Question: Consider a equilibrium system: H2c + 9) *+ 2H1 = 13.0 at 298. 1 1.20 ml of H and 1.20 of 13 are placed in
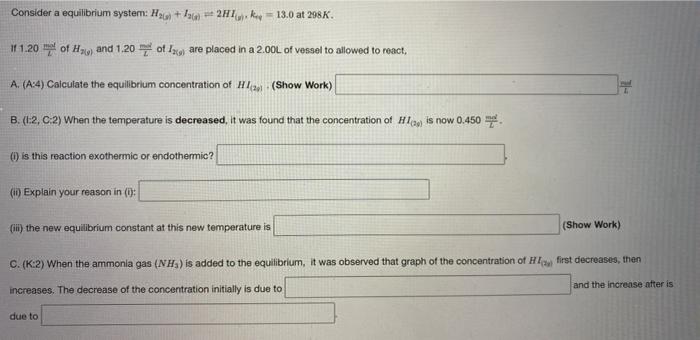
Consider a equilibrium system: H2c + 9) *+ 2H1 = 13.0 at 298. 1 1.20 ml of H and 1.20 of 13 are placed in a 2.00L of vessel to allowed to react, A. (:4) Calculate the equilibrium concentration of H12 (Show Work) B. (1:2, 0:2) When the temperature is decreased, it was found that the concentration of law is now 0.450 () is this reaction exothermic or endothermic? (1) Explain your reason in (0: (iii) the new equilibrium constant at this new temperature is (Show Work) C. (K:2) When the ammonia gas (NH) is added to the equilibrium, it was observed that graph of the concentration of first decreases, then and the increase after is increases. The decrease of the concentration initially is due to due to
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


