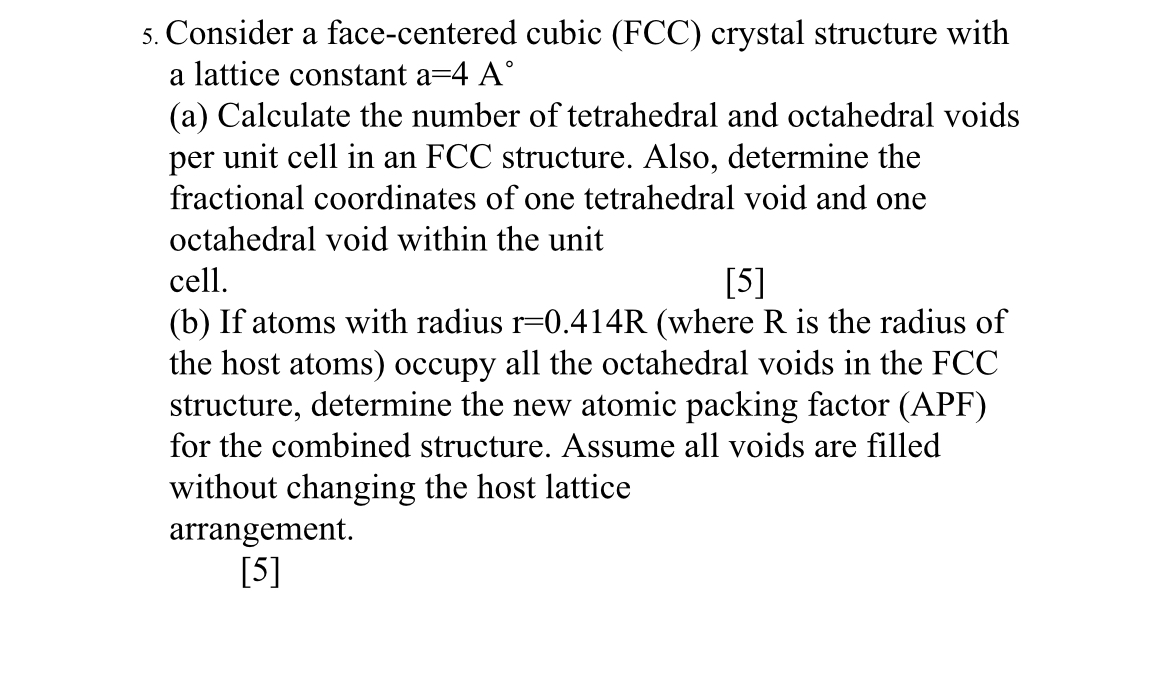
Question: Consider a face - centered cubic ( FCC ) crystal structure with a lattice constant a = 4 A ( a ) Calculate the number
Consider a facecentered cubic FCC crystal structure with a lattice constant
a Calculate the number of tetrahedral and octahedral voids per unit cell in an FCC structure. Also, determine the fractional coordinates of one tetrahedral void and one octahedral void within the unit cell.
b If atoms with radius where R is the radius of the host atoms occupy all the octahedral voids in the FCC structure, determine the new atomic packing factor APF for the combined structure. Assume all voids are filled without changing the host lattice arrangement.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


