Question: Consider a gas expansion process (shown in scheme below). Two 510 kg weights sit on the piston. The piston is assumed to be weightless and
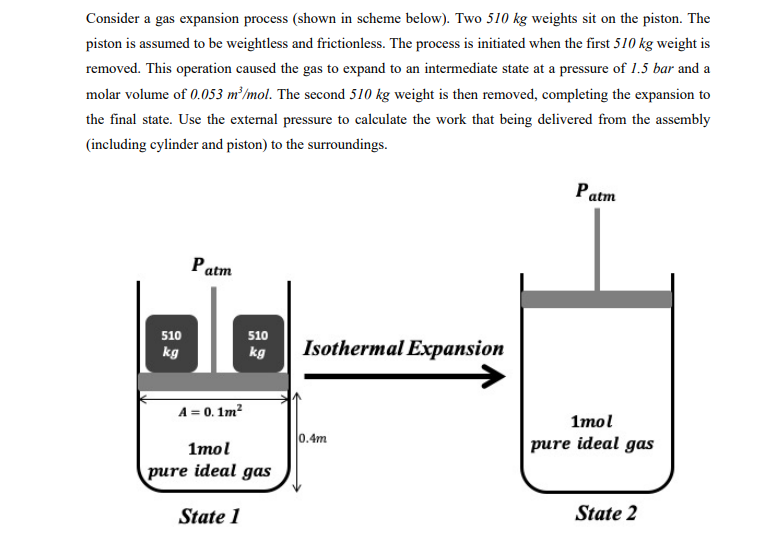
Consider a gas expansion process (shown in scheme below). Two 510 kg weights sit on the piston. The piston is assumed to be weightless and frictionless. The process is initiated when the first 510 kg weight is removed. This operation caused the gas to expand to an intermediate state at a pressure of 1.5 bar and a molar volume of 0.053 m/mol. The second 510 kg weight is then removed, completing the expansion to the final state. Use the external pressure to calculate the work that being delivered from the assembly (including cylinder and piston) to the surroundings. Patm Patm 510 kg 510 kg Isothermal Expansion A = 0. 1m2 0.4m 1mol pure ideal gas 1mol pure ideal gas State 1 State 2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


