Question: Consider a hypothetical atom with electron Daniel whose possible energy levels are shown below on the next page. The energy (in arbitrary units) of

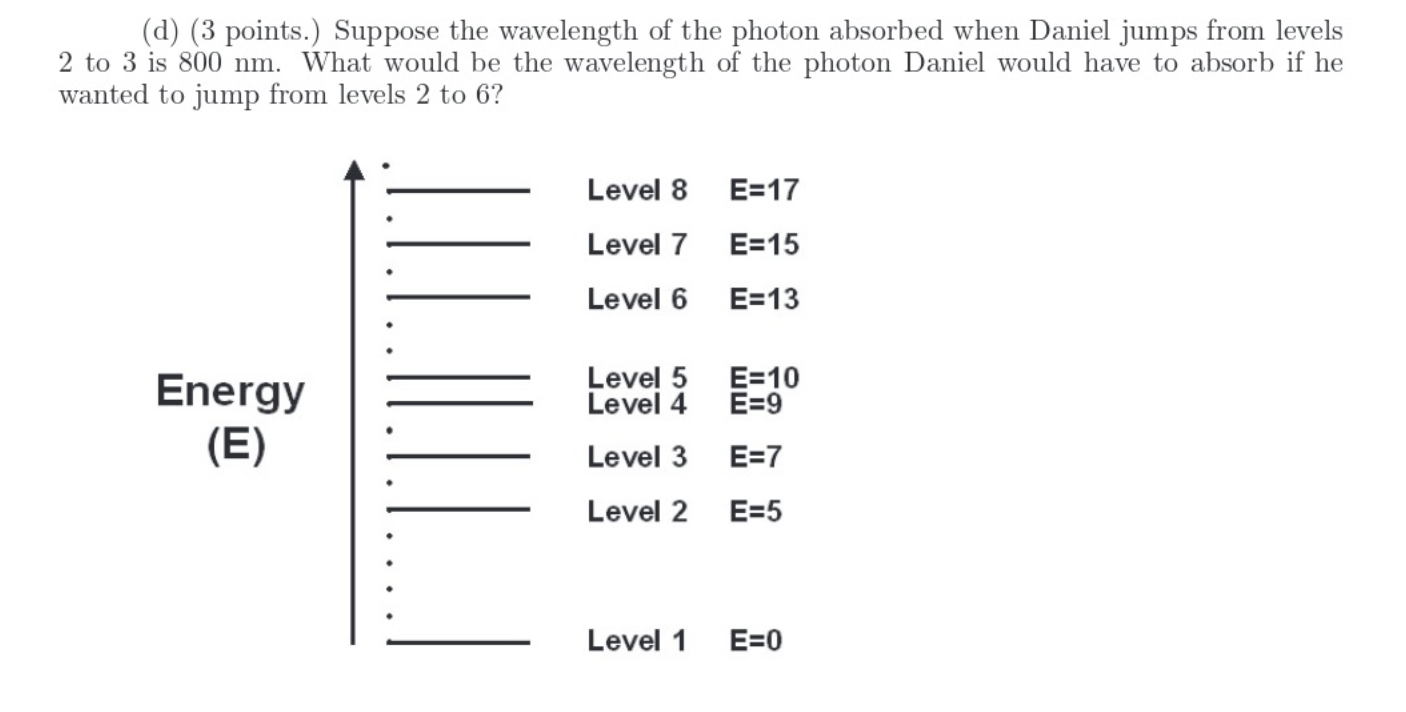
Consider a hypothetical atom with electron Daniel whose possible energy levels are shown below on the next page. The energy (in arbitrary units) of each higher level is given relative to the ground (lowest) state. (a) (2 points.) When Daniel jumps from levels 1 to 3, he must absorb a photon having a specific energy. Identify another electronic transition that requires the absorption of a photon with the same energy. (b) (2 points.) Suppose Daniel jumps from levels 6 to 3, followed by levels 3 to 2. What is the ratio of the energies of the photons emitted? (c) (2+1=3 points.) If Daniel subsequently jumps from levels 2 to 1, can the emitted photon be absorbed by another identical atom whose electron Rav happens to be in level 5? Explain carefully. (d) (3 points.) Suppose the wavelength of the photon absorbed when Daniel jumps from levels 2 to 3 is 800 nm. What would be the wavelength of the photon Daniel would have to absorb if he wanted to jump from levels 2 to 6? Level 8 E=17 Level 7 E=15 Level 6 E=13 Energy (E) Level 5 Level 4 E=10 E=9 Level 3 E=7 Level 2 E=5 Level 1 E=0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


