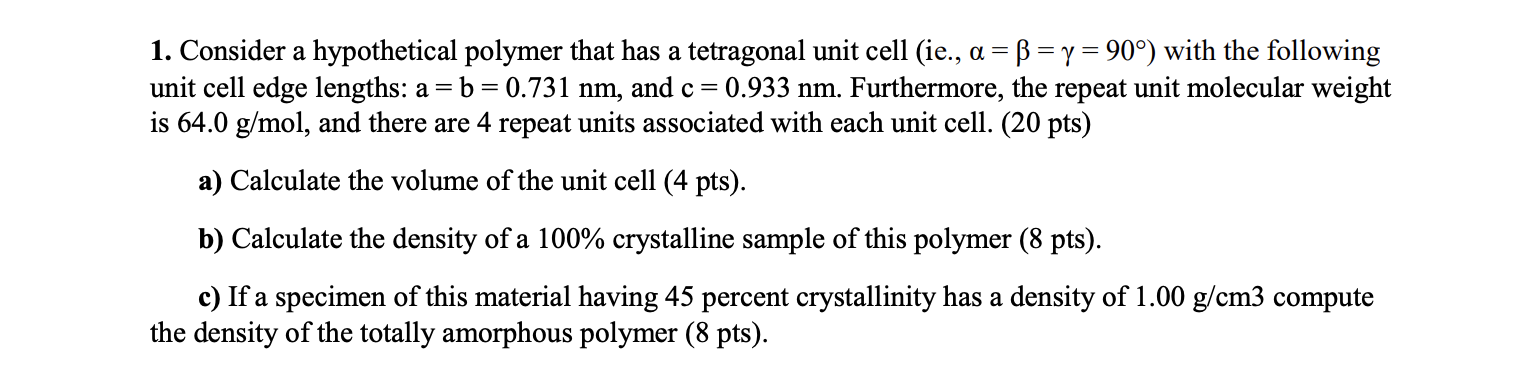
Question: Consider a hypothetical polymer that has a tetragonal unit cell ( ie . , = = = 9 0 ) with the following unit cell
Consider a hypothetical polymer that has a tetragonal unit cell ie with the following
unit cell edge lengths: and Furthermore, the repeat unit molecular weight
is and there are repeat units associated with each unit cell. pts
a Calculate the volume of the unit cell pts
b Calculate the density of a crystalline sample of this polymer pts
c If a specimen of this material having percent crystallinity has a density of compute
the density of the totally amorphous polymer pts
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


