Question: Consider a material that can exist in two distinct phases, and , depending on the pressure and temperature. At any point along the coexistence line
Consider a material that can exist in two distinct phases, and , depending on the pressure and temperature. At any point along the coexistence line in the phase diagram, the Gibbs free energy of the two phases is the same: G (P, T) = G (P, T). In this problem, you will derive some useful properties of the slope of the coexistence line.
a)The coexistence line is a function P(T). Imagine the system is at coexistence. Write down the expression for how G changes if you make a small change in pressure and temperature, and do the same for G. If you want to stay on the coexistence line after such a change, you cannot change P and T independently; you have to change them in a specific ratio. Calculate this ratio dP/dT in terms of the entropies and volumes of the two phases.
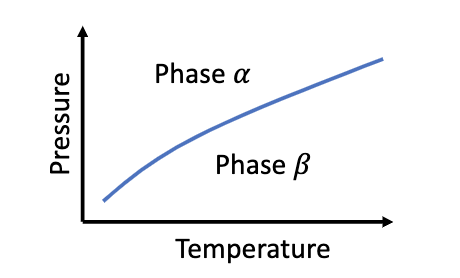
Phase a Pressure Phase Temperature
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


