Question: Phase a 1. Consider a material that can exist in two distinct phases, a and B, depending on the pressure and temperature. At any point
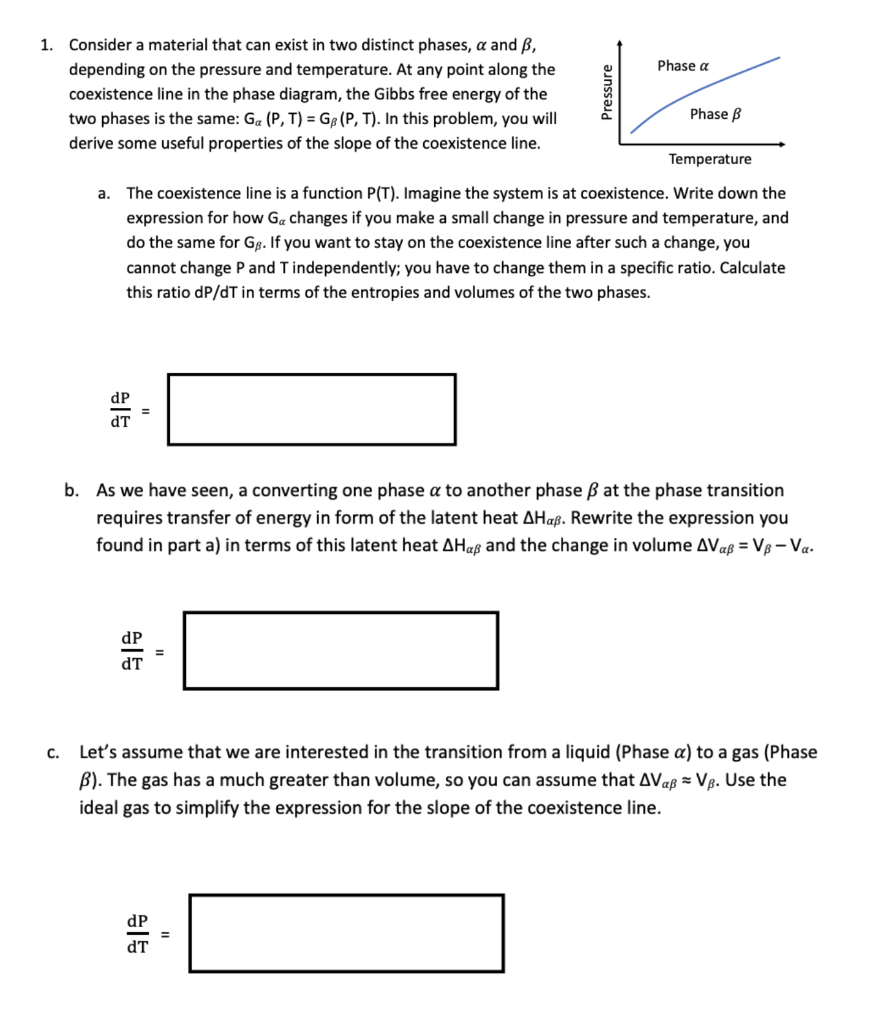
Phase a 1. Consider a material that can exist in two distinct phases, a and B, depending on the pressure and temperature. At any point along the coexistence line in the phase diagram, the Gibbs free energy of the two phases is the same: Ga (P, T) = GB(P, T). In this problem, you will derive some useful properties of the slope of the coexistence line. Pressure Phase B Temperature a. The coexistence line is a function P(T). Imagine the system is at coexistence. Write down the expression for how Ga changes if you make a small change in pressure and temperature, and do the same for Gs. If you want to stay on the coexistence line after such a change, you cannot change P and Tindependently; you have to change them in a specific ratio. Calculate this ratio dP/dT in terms of the entropies and volumes of the two phases. dP 1 = dT b. As we have seen, a converting one phase a to another phase B at the phase transition requires transfer of energy in form of the latent heat AHa. Rewrite the expression you found in part a) in terms of this latent heat AHa and the change in volume Ava = V8-Va. dP dT C. Let's assume that we are interested in the transition from a liquid (Phase a) to a gas (Phase B). The gas has a much greater than volume, so you can assume that Ava = VB. Use the ideal gas to simplify the expression for the slope of the coexistence line. $1 dP dT
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


