Consider a non-ideal gas that follows the Van der Waals equation of state: RT a v-b...
Fantastic news! We've Found the answer you've been seeking!
Question:
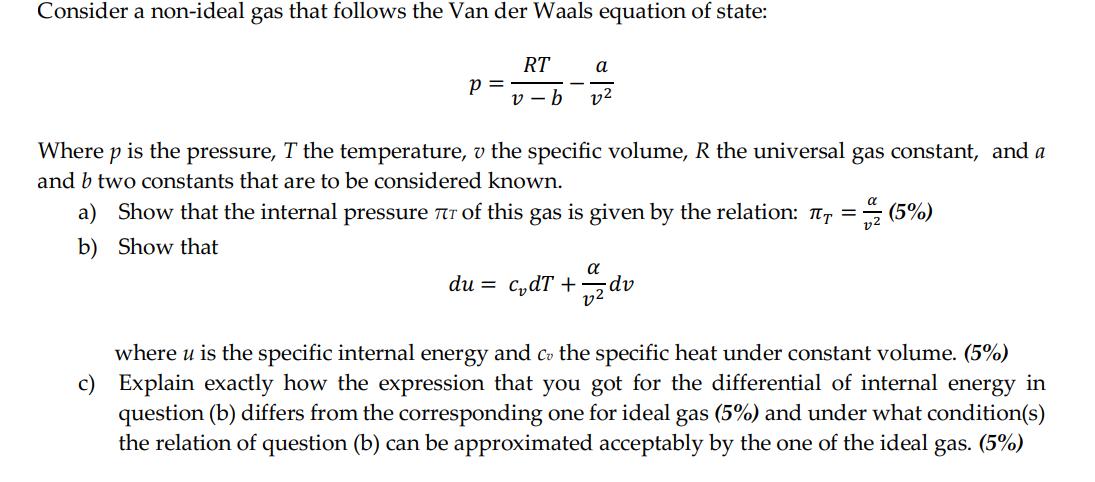
Transcribed Image Text:
Consider a non-ideal gas that follows the Van der Waals equation of state: RT a v-b 22 P = Where p is the pressure, T the temperature, v the specific volume, R the universal gas constant, and a and b two constants that are to be considered known. a) Show that the internal pressure 7 of this gas is given by the relation: π = (5%) b) Show that α du = c₂dT + 2 dv where u is the specific internal energy and co the specific heat under constant volume. (5%) c) Explain exactly how the expression that you got for the differential of internal energy in question (b) differs from the corresponding one for ideal gas (5%) and under what condition(s) the relation of question (b) can be approximated acceptably by the one of the ideal gas. (5%) Consider a non-ideal gas that follows the Van der Waals equation of state: RT a v-b 22 P = Where p is the pressure, T the temperature, v the specific volume, R the universal gas constant, and a and b two constants that are to be considered known. a) Show that the internal pressure 7 of this gas is given by the relation: π = (5%) b) Show that α du = c₂dT + 2 dv where u is the specific internal energy and co the specific heat under constant volume. (5%) c) Explain exactly how the expression that you got for the differential of internal energy in question (b) differs from the corresponding one for ideal gas (5%) and under what condition(s) the relation of question (b) can be approximated acceptably by the one of the ideal gas. (5%)
Expert Answer:

Related Book For 

Posted Date:
Students also viewed these general management questions
-
On June 1 of the current year, Chris Bates established a business to manage rental property. The following transactions were completed during June: a. Opened a business bank account with a deposit of...
-
The overall goal of this problem is to compute the PV and PT equilibrium diagramsfor a single component fluid described by the van derWaals equation of state. Let us recall the key things we need to...
-
The van der Waals equation of state, an approximate representation of the behavior of gases at high pressure, is given by Where a and b are constants having different values for different gases. (In...
-
Prepare budgetary and proprietary journal entries to record the following year- end adjustments: 1. An accrual of $ 60,000 was made for salaries earned the last week of September, to be paid in...
-
Which planets have a more-than-one-Earth-year period: planets nearer than Earth to the Sun, or planets farther from the Sun than Earth?
-
Donovan & Parents produces soccer shorts and jerseys for youth leagues. Most of the production is done by machine. Data on operations and costs for March follow: Management asks the firm's cost...
-
On November 15, 2010, Chandlers Department Store purchased $5,100 of inventory on account from one of its suppliers. The terms were 3/15, n/45, FOB shipping point. On November 18 Chandlers Department...
-
On January 2, 2008, P Company, a U.S.-based company, acquired for 2,000,000 francs an 80% interest in SFr Company. On January 2, 2008, SFr Company reported a retained earnings balance of 480,000...
-
A company manufactures a liquid product called Crystal. The basic ingredients are put into process in Department 1. In Department 2, other materials are added that increase the number of units being...
-
On December 31, 2023, Stilton Service Companys year-end, the unadjusted trial balance included the following items: Required 1. Prepare the adjusting entry on the books of Stilton Service Company to...
-
Write a brief note no Mobile Communication System in which you need to address the following: Introduction Analysis of UNICEFs Mobile Communication System UNICEF Mobile Communication Applications...
-
b. The hydroelectric facility that has been proposed will consist of a turbine with an efficiency of 90.4%. The facility will be located at a vertical depth of 15.2 m below the riverbed. The river...
-
Walmart buys each bottle of Tylenol for $3.00. Walmart sells 624 bottles through a store every year and demand is relatively constant throughout the year. Walmart can place orders for more Tylenol...
-
Consider the following normal distribution curve modeling the daily demand of a continuous review inventory system. Reference: Bell Curve - dL Which is the following is correct with respect to R...
-
Determine the resultant and indicate the angle or slope for the right-angle force system shown in Figure 4. 80 N 150 N FIGURE 4
-
Willow Inc. has provided the following information: Standards: Direct materials Direct labor Variable overhead Fixed overhead Total Per unit 10 lbs $2.80/lb $28.00 2 hours @ $16.50/hour 33.00 2 hours...
-
15 The concentration of hole-electron pairs in pure silicon at T-300 K is 7x 10 per cubic metre. Antimony is doped into silicon in a proportion of 1 atom in 10 atoms. Assuming that half of the...
-
Clark, PA, has been engaged to perform the audit of Kent Ltd.s financial statements for the current year. Clark is about to commence auditing Kents employee pension expense. Her preliminary enquiries...
-
Prove property (3). u X v = -(v x u) (3)
-
Write a few paragraphs outlining your strategy for establishing identities.
-
Graph each function. Be sure to label key points and show at least two cycles. Use the graph to determine the domain and the range of each function. y = cos(-2x)
-
Let a be the number such that the area to the right of z = a is 0.21. Without using a table or technology, find the area between z = a and z = a.
-
Your medical terminology instructor listed the following grades for the class out of a 75-point test: 34, 36, 41 , 43, 44,49,50, 55,57, 60,64, 66, 67,67, 67,68,68,69, 70, 73 a. Find the 90th...
-
From the following list of number of discharges each day in September, compute the mean, median, mode, and range. Round the mean and median to one decimal point. University Hospital Number of...

Study smarter with the SolutionInn App


