Question: Consider a nonisothermal batch reactor which is operated adiabatically. The reactor contains a liquid reaction mixture in which the following reaction occurs. A(l)rP(l) where r=kCa
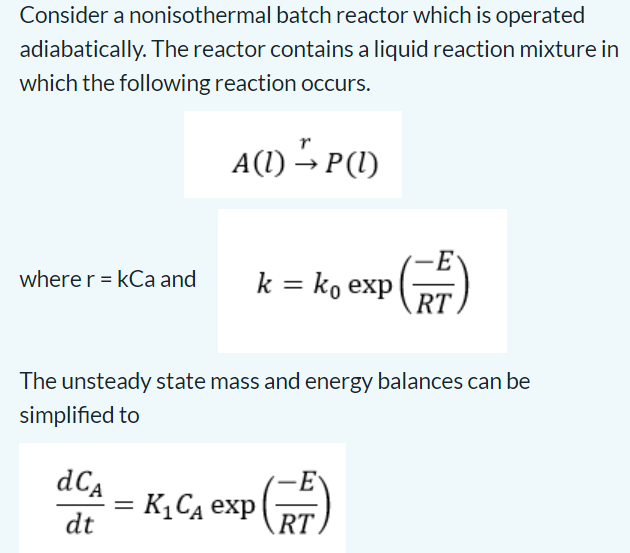
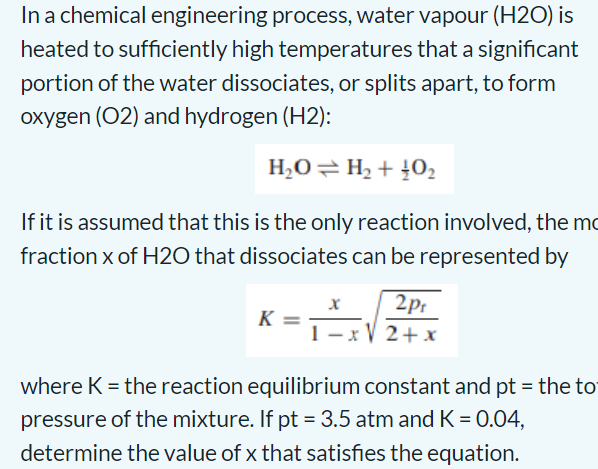
Consider a nonisothermal batch reactor which is operated adiabatically. The reactor contains a liquid reaction mixture in which the following reaction occurs. A(l)rP(l) where r=kCa and k=k0exp(RTE) The unsteady state mass and energy balances can be simplified to dtdCA=K1CAexp(RTE) In a chemical engineering process, water vapour (H2O) is heated to sufficiently high temperatures that a significant portion of the water dissociates, or splits apart, to form oxygen (O2) and hydrogen (H2) : H2OH2+21O2 If it is assumed that this is the only reaction involved, the m fraction x of H2O that dissociates can be represented by K=1xx2+x2pt where K = the reaction equilibrium constant and pt = the to pressure of the mixture. If pt=3.5atm and K=0.04, determine the value of x that satisfies the equation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


