Question: Consider a square space lattice where A,B,C,D,E,F are drawn unit cells. Answer the following: a) Which of the two-dimensional unit cells shown on the diagram



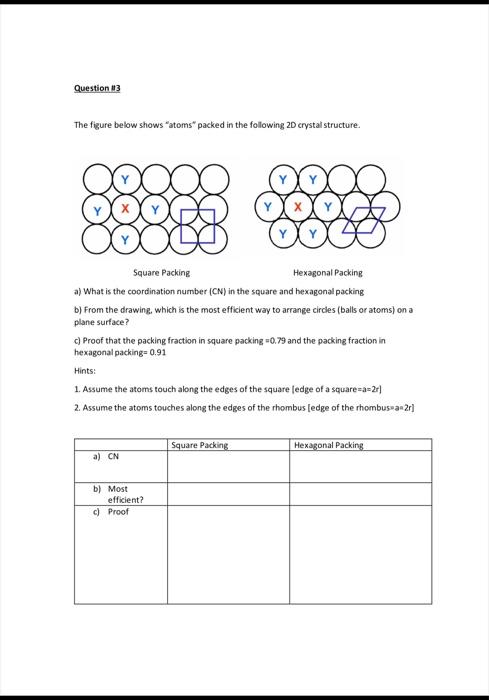

Consider a square space lattice where A,B,C,D,E,F are drawn unit cells. Answer the following: a) Which of the two-dimensional unit cells shown on the diagram below are primitive and which are not? Explain your answer. b) Which out of the these unit cells (A,B,C,D,E,F) is a proper unit cell for this lattice? Explain why? The atomic arrangement of 20 lattices (1) and (2), are given below: a) Draw the proper unit cell in each lattice b) Write the lattice parameters c) What is the crystal system for each lattice The figure below shows "atoms" packed in the following 2D crystal structure. a) What is the coordination number (CN) in the square and hexagonal packing b) from the drawing. which is the most efficient way to arrange circles (balls or atoms) on a plane surface? c) Proof that the packing fraction in square packing =0.79 and the packing fraction in hexagonal packing =0.91 Hints: 1. Assume the atoms touch along the edges of the square [edge of a square =a=2r ] 2. Assume the atoms touches along the edges of the rhombus [edge of the rhombuswan 2r] Question \#A Graphene is a two-dimensional lattice which is built by a continuous arrangement of regular hexagons of sp2 hybridized carbon atoms (see below). The distance between nearest neighbor atoms is 0.14nm. (a) What is the Bravais lattice of graphene? (b) Assuming that the unit cell is a hexagon of 6 carbon atoms, how many atoms of carbon are there in each unit cell
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


