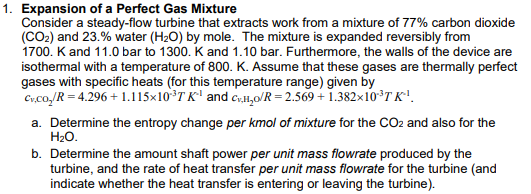
Question: Consider a steady - flow turbine that extracts work from a mixture of 7 7 % carbon dioxide Expansion of a Perfect Gas Mixture Consider
Consider a steadyflow turbine that extracts work from a mixture of carbon dioxide Expansion of a Perfect Gas Mixture
Consider a steadyflow turbine that extracts work from a mixture of carbon dioxide
and water by mole. The mixture is expanded reversibly from
K and bar to K and bar. Furthermore, the walls of the device are
isothermal with a temperature of K Assume that these gases are thermally perfect
gases with specific heats for this temperature range given by
and
a Determine the entropy change per kmol of mixture for the and also for the
b Determine the amount shaft power per unit mass flowrate produced by the
turbine, and the rate of heat transfer per unit mass flowrate for the turbine and
indicate whether the heat transfer is entering or leaving the turbine
CO and water HO by mole. The mixture is expanded reversibly from
K and bar to K and bar. Furthermore, the walls of the device are
isothermal with a temperature of K Assume that these gases are thermally perfect
gases with specific heats for this temperature range given by
cvCO
R T K
and cvH
OR T K
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


