Question: Consider a system A (lying at thermal equilibrium at temperature T) consisting of 2N- non- interacting distinguishable particles constrained to move on the surface
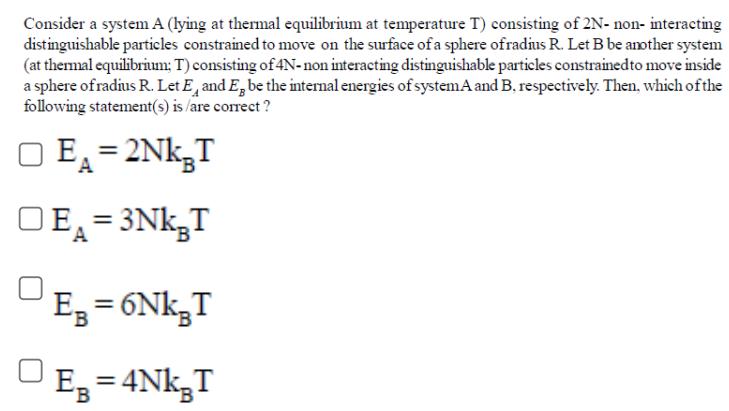
Consider a system A (lying at thermal equilibrium at temperature T) consisting of 2N- non- interacting distinguishable particles constrained to move on the surface of a sphere of radius R. Let B be another system (at thermal equilibrium; T) consisting of 4N-non interacting distinguishable particles constrained to move inside a sphere of radius R. Let E and E, be the internal energies of system A and B. respectively. Then, which of the following statement(s) is/are correct? O EA=2NkT DEA=3NkT E = 6Nk T EB B=4NkT
Step by Step Solution
3.38 Rating (157 Votes )
There are 3 Steps involved in it
The detailed ... View full answer

Get step-by-step solutions from verified subject matter experts


