Question: Consider a system that undergoes a phase change from phase a to phase b at equilibrium temperature T and equilibrium pressure p . At these
Consider a system that undergoes a phase change from phase a to phase b at equilibrium temperature T and equilibrium pressure p
and equilibrium pressure p . At these equilibrium conditions, the heat absorbed per mole of material undergoing this transition is q
. At these equilibrium conditions, the heat absorbed per mole of material undergoing this transition is q , and there is a molar volume change of
, and there is a molar volume change of 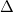 V
V ,m. The molar heat capacities for the
,m. The molar heat capacities for the 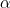 and
and 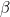 phases are Cp,m,
phases are Cp,m,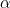 and Cp,m,
and Cp,m,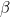 ; they are independent of temperature.
; they are independent of temperature.
A.) Write a thermodynamic cycle that would allow you to calculate the equilibrium transition temperature corresponding to a pressure of 1000 bar. Use the cycle to write an equation that could in principle be solved to calculate T at 1000 bar.
at 1000 bar.
Transcribed image text
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


