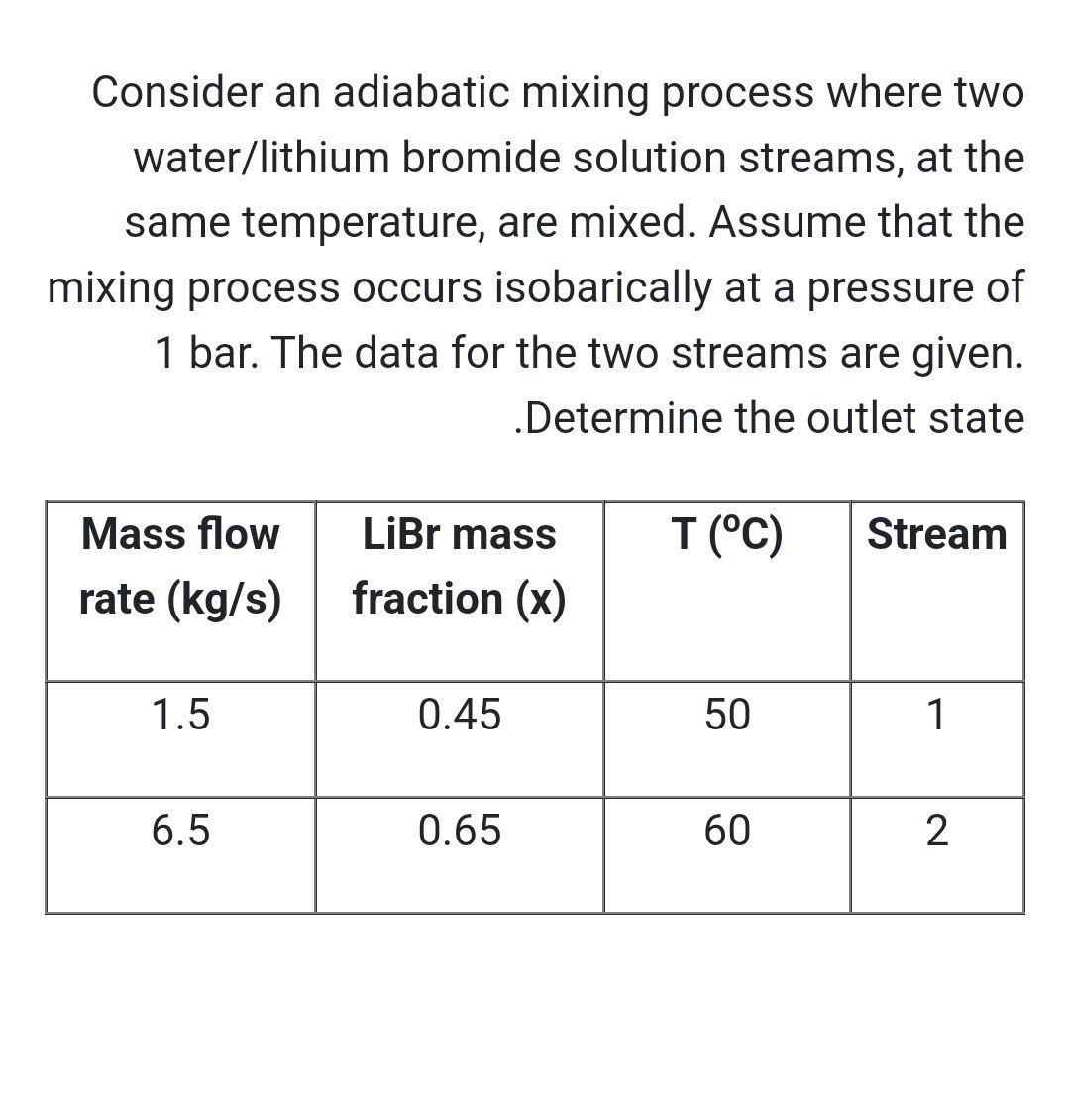
Question: Consider an adiabatic mixing process where two water / lithium bromide solution streams, at the same temperature, are mixed. Assume that the mixing process occurs
Consider an adiabatic mixing process where two waterlithium bromide solution streams, at the same temperature, are mixed. Assume that the mixing process occurs isobarically at a pressure of bar. The data for the two streams are given.
Determine the outlet state
tabletableMass flowrate kgstableLiBr massfraction xStream
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


