Question: Consider an exothermic reversible reaction with two reactants and two products: A+BC+D The reaction takes place in a CSTR that has two feed streams, FA0
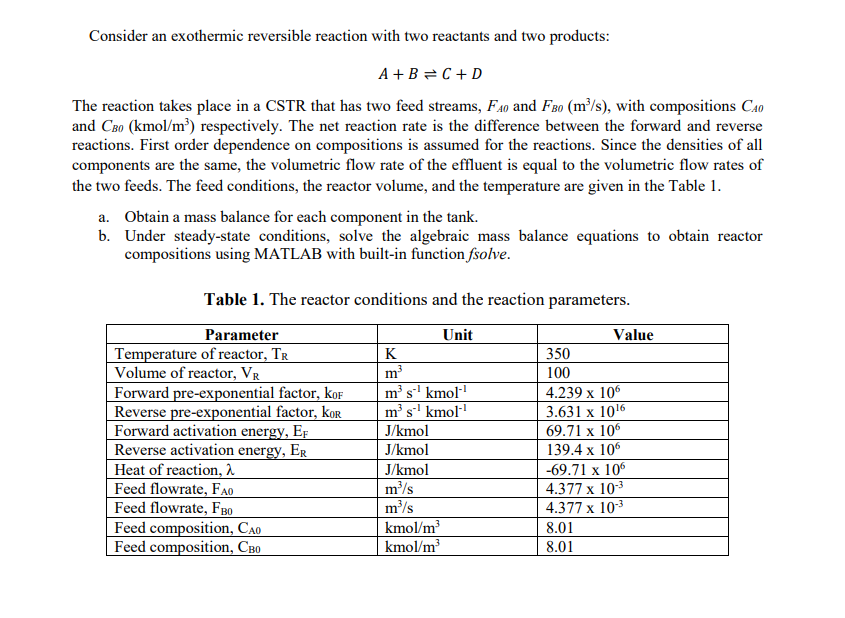
Consider an exothermic reversible reaction with two reactants and two products: A+BC+D The reaction takes place in a CSTR that has two feed streams, FA0 and FBO(m3/s), with compositions CAO and CBO(kmol/m3) respectively. The net reaction rate is the difference between the forward and reverse reactions. First order dependence on compositions is assumed for the reactions. Since the densities of all components are the same, the volumetric flow rate of the effluent is equal to the volumetric flow rates of the two feeds. The feed conditions, the reactor volume, and the temperature are given in the Table 1 . a. Obtain a mass balance for each component in the tank. b. Under steady-state conditions, solve the algebraic mass balance equations to obtain reactor compositions using MATLAB with built-in function fsolve. Table 1. The reactor conditions and the reaction parameters
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


