Question: - Consider that the metal and various complexing ligands beyond hydroxide are present at abundances of 103 M. For each combination of metal and ligands,
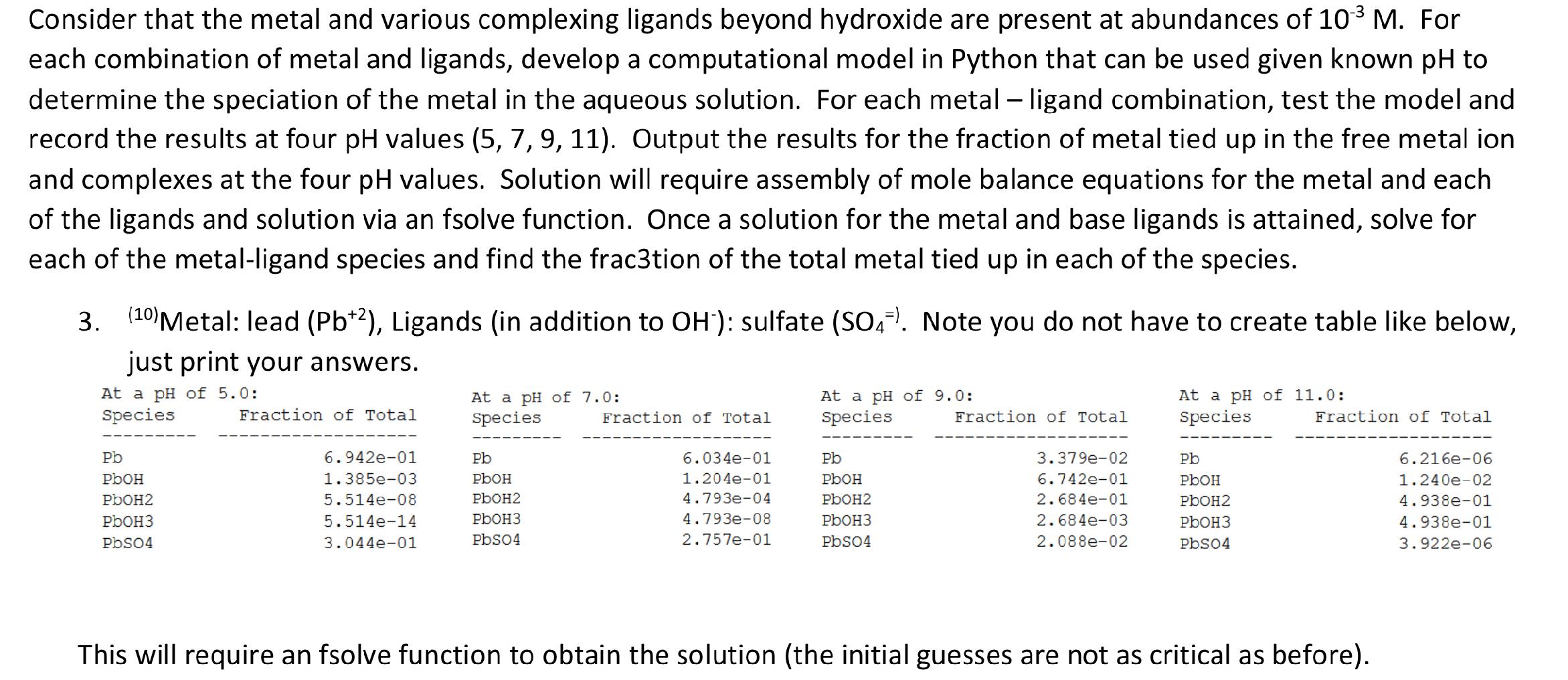
- Consider that the metal and various complexing ligands beyond hydroxide are present at abundances of 103 M. For each combination of metal and ligands, develop a computational model in Python that can be used given known pH to determine the speciation of the metal in the aqueous solution. For each metal - ligand combination, test the model and record the results at four pH values (5, 7, 9, 11). Output the results for the fraction of metal tied up in the free metal ion and complexes at the four pH values. Solution will require assembly of mole balance equations for the metal and each of the ligands and solution via an fsolve function. Once a solution for the metal and base ligands is attained, solve for each of the metal-ligand species and find the frac3tion of the total metal tied up in each of the species. 1 3. (10) Metal: lead (Pb+2), Ligands (in addition to OH"): sulfate (S04!. Note you do not have to create table like below, just print your answers. At a pH of 5.0: Species Fraction of Total At a pH of 7.0: Species Fraction of Total At a pH of 9.0: Species Fraction of Total At a pH of 11.0: Species Fraction of Total Pb PbOH PbOH2 PbOH3 PbSO4 6.942e-01 1.385e-03 5.514e-08 5.514e-14 3.044e-01 Pb PLOH PbOH2 PbOH3 PbSO4 6.034e-01 1.204e-01 4.793e-04 4.793e-08 2.757e-01 Pb PbOH PbOH2 PbOH3 PbSO4 3.379e-02 6.742e-01 2.684e-01 2.684e-03 2.088e-02 Pb PbOH PbOH2 PbOH3 Pbs04 6.216e-06 1.240e-02 4.938e-01 4.938e-01 3.922e-06 This will require an fsolve function to obtain the solution (the initial guesses are not as critical as before)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


