Question: problem1 The X-ray data below was taken from an unknown metal, you goal is to find out what metal the sample was made of following
problem1
The X-ray data below was taken from an unknown metal, you goal is to find out what metal the sample was made of following the steps below. The wavelength of the Copper radiation was 1.5405 . Provide answers for each question below.
a. Justify why this x-ray pattern is from a FCC metal (remember selection rules)
b. Calculate the interplanar spacing, d, by choosing one of the diffraction peaks from the data and using Braggs Law.
c. Calculate the lattice parameter using the interplanar spacing equation from class.
d. Calculate the radius of the atom assuming hard spheres in contact
e. Identify the metal based on its crystal structure and lattice parameter
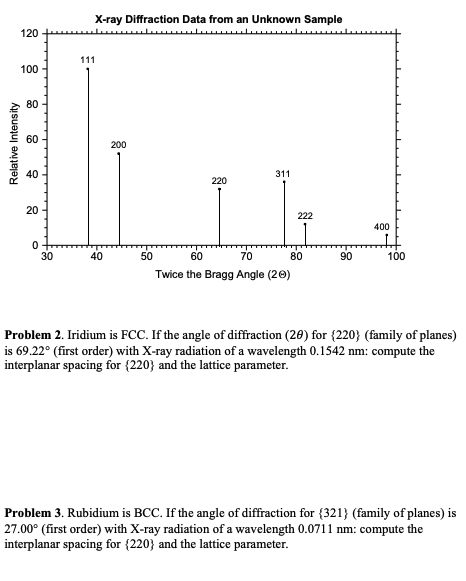
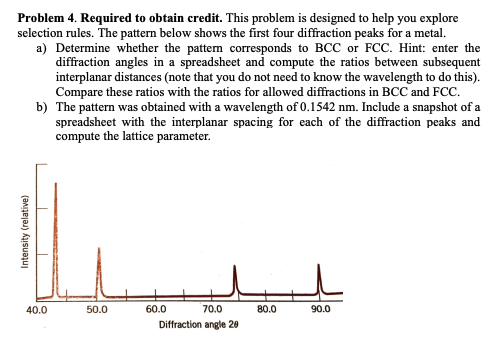
X-ray Diffraction Data from an Unknown Sample 120 111 100 80 Relative Intensity 60 200 40 311 220 20 222 0 30 400 LE 100 40 90 50 60 70 80 Twice the Bragg Angle (20) Problem 2. Iridium is FCC. If the angle of diffraction (20) for (220) (family of planes) is 69.22 (first order) with X-ray radiation of a wavelength 0.1542 nm: compute the interplanar spacing for (220) and the lattice parameter. Problem 3. Rubidium is BCC. If the angle of diffraction for {321) (family of planes) is 27.00 (first order) with X-ray radiation of a wavelength 0.0711 nm: compute the interplanar spacing for (220) and the lattice parameter. Problem 4. Required to obtain credit. This problem is designed to help you explore selection rules. The pattern below shows the first four diffraction peaks for a metal. a) Determine whether the pattem corresponds to BCC or FCC. Hint: enter the diffraction angles in a spreadsheet and compute the ratios between subsequent interplanar distances (note that you do not need to know the wavelength to do this). Compare these ratios with the ratios for allowed diffractions in BCC and FCC. b) The pattern was obtained with a wavelength of 0.1542 nm. Include a snapshot of a spreadsheet with the interplanar spacing for each of the diffraction peaks and compute the lattice parameter. Intensity (relative) 40.0 50.0 80.0 90.0 60.0 70.0 Diffraction angle 29
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


