Question: Consider the Carnot cycle shown below: . Starting from (a), the gas is compressed at constant temperature to (b) . The gas is then compressed
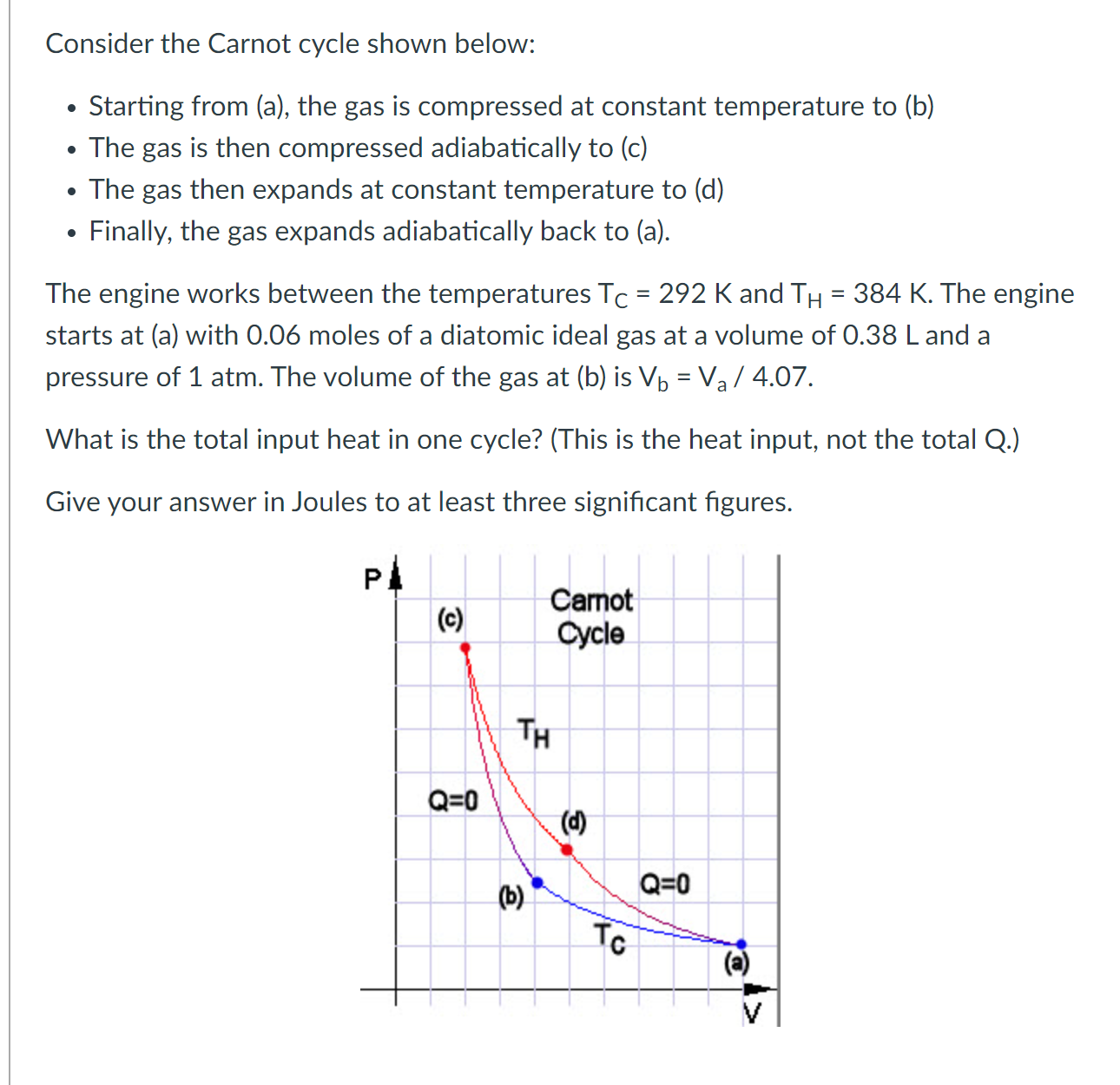
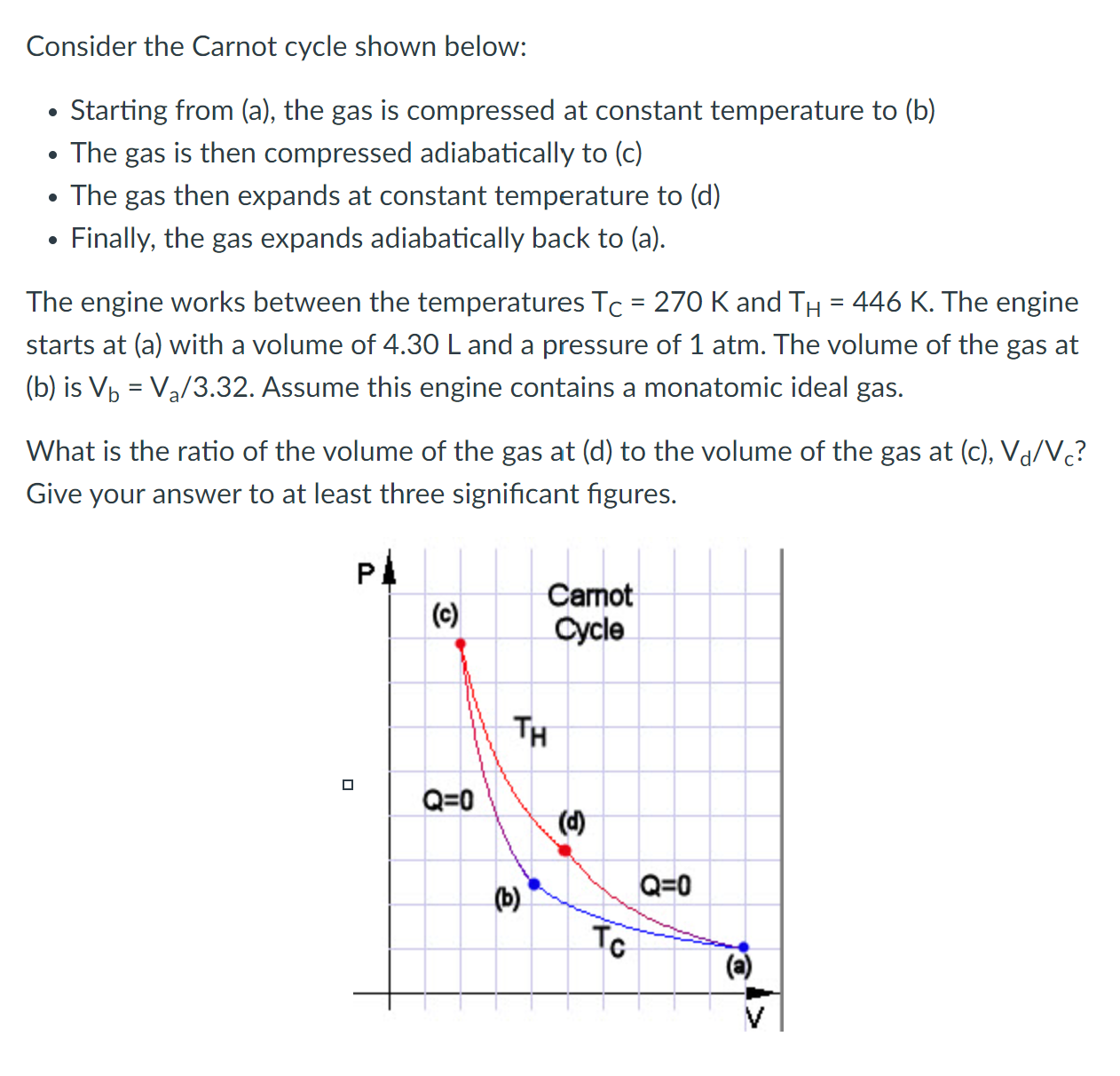
Consider the Carnot cycle shown below: . Starting from (a), the gas is compressed at constant temperature to (b) . The gas is then compressed adiabatically to (c) . The gas then expands at constant temperature to (d) . Finally, the gas expands adiabatically back to (a). The engine works between the temperatures Tc = 292 K and TH = 384 K. The engine starts at (a) with 0.06 moles of a diatomic ideal gas at a volume of 0.38 L and a pressure of 1 atm. The volume of the gas at (b) is Vb = Va / 4.07. What is the total input heat in one cycle? (This is the heat input, not the total Q.) Give your answer in Joules to at least three signicant gures. Pl l (c) Carnot cycle Consider the Carnot cycle shown below: . Starting from (a), the gas is compressed at constant temperature to (b) . The gas is then compressed adiabatically to (c) . The gas then expands at constant temperature to (d) . Finally, the gas expands adiabatically back to (a). The engine works between the temperatures Tc = 270 K and TH = 446 K. The engine starts at (a) with a volume of 4.30 L and a pressure of 1 atm. The volume of the gas at (b) is Vb = Va/3.32. Assume this engine contains a monatomic ideal gas. What is the ratio of the volume of the gas at (d) to the volume of the gas at (c), Vd/VC? Give your answer to at least three signicant gures. Pl | to) 311\" O
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


