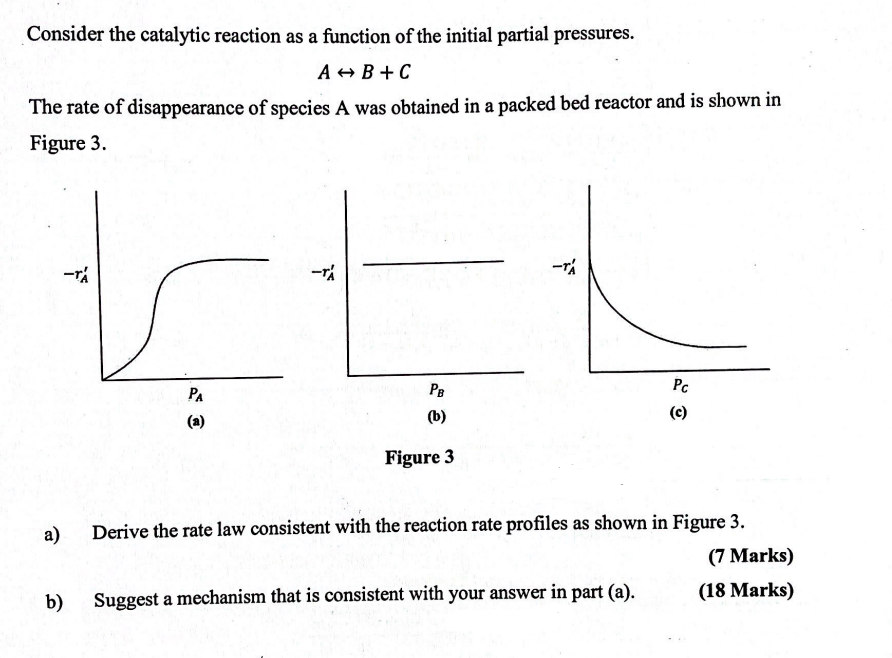
Question: Consider the catalytic reaction as a function of the initial partial pressures, A + B - > CConsider the catalytic reaction as a function of
Consider the catalytic reaction as a function of the initial partial pressures,
AB CConsider the catalytic reaction as a function of the initial partial pressures.
AharrB
The rate of disappearance of species A was obtained in a packed bed reactor and is shown in
Figure
a
b
Figure
a Derive the rate law consistent with the reaction rate profiles as shown in Figure
b Suggest a mechanism that is consistent with your answer in part a
The rate of disappearance of species A was obtained in a differential reactor and the data shown below. Propose a rate law that matches the data above.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


