Question: Consider the equilibrium reaction 2A+B 4C After multiplying the reaction by a factor of 2, what is the new equilibrium equation? 0 Drag the
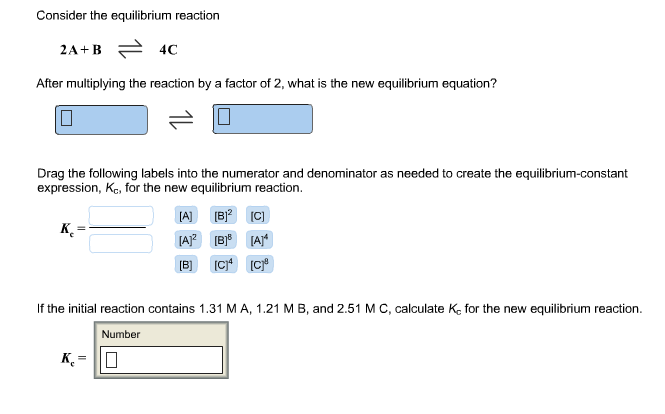
Consider the equilibrium reaction 2A+B 4C After multiplying the reaction by a factor of 2, what is the new equilibrium equation? 0 Drag the following labels into the numerator and denominator as needed to create the equilibrium-constant expression, Kc, for the new equilibrium reaction. K [A] [B][C] [A] [B] [A]* [B] [C]4 [C] If the initial reaction contains 1.31 MA, 1.21 MB, and 2.51 M C, calculate K, for the new equilibrium reaction. Number K=
Step by Step Solution
There are 3 Steps involved in it
The given equilibrium reaction is as follows 2AB 4C As ... View full answer

Get step-by-step solutions from verified subject matter experts


