Question: Consider the equilibrium reaction. 3A+B=2C After multiplying the reaction by a factor of 2 , what is the new equilibrium equation? new equilibrium equation: Incorrect
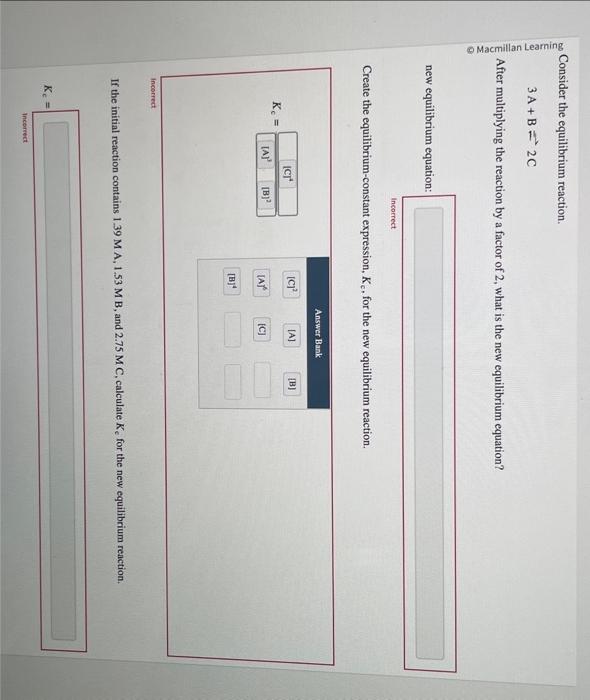
Consider the equilibrium reaction. 3A+B=2C After multiplying the reaction by a factor of 2 , what is the new equilibrium equation? new equilibrium equation: Incorrect Create the equilibrium-constant expression, Kc, for the new equilibrium reaction. Kc= Incorrect. If the initial reaction contains 1.39MA,1.53MB, and 2.75MC, calculate Kc for the new equilibrium renction
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


