Question: Consider the first-order gas phase reaction (A B) taking place on the inner surface of a straight cylindrical catalyst pore. Assume that the catalytic reaction
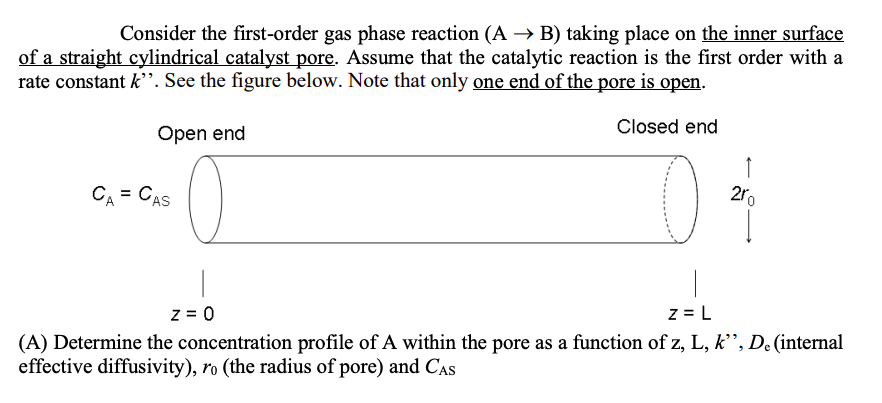
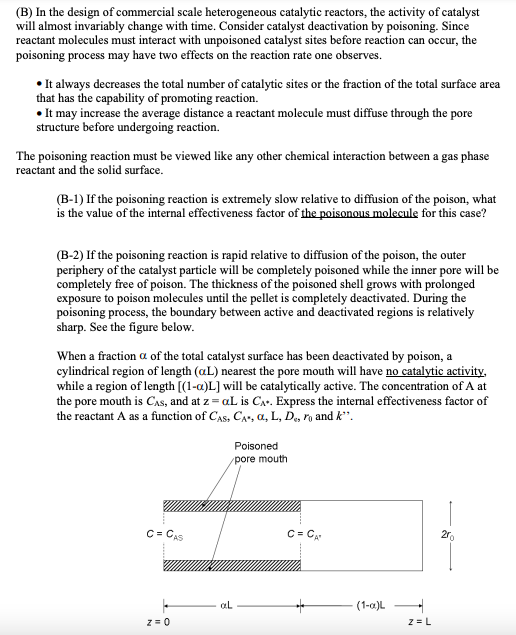
Consider the first-order gas phase reaction (A B) taking place on the inner surface of a straight cylindrical catalyst pore. Assume that the catalytic reaction is the first order with a rate constant k". See the figure below. Note that only one end of the pore is open. Open end Closed end CA = CAS z = 0 Z=L (A) Determine the concentration profile of A within the pore as a function of z, L, k", De (internal effective diffusivity), ro (the radius of pore) and CAS 200 (B) In the design of commercial scale heterogeneous catalytic reactors, the activity of catalyst will almost invariably change with time. Consider catalyst deactivation by poisoning. Since reactant molecules must interact with unpoisoned catalyst sites before reaction can occur, the poisoning process may have two effects on the reaction rate one observes. It always decreases the total number of catalytic sites or the fraction of the total surface area that has the capability of promoting reaction. It may increase the average distance a reactant molecule must diffuse through the pore structure before undergoing reaction. The poisoning reaction must be viewed like any other chemical interaction between a gas phase reactant and the solid surface. (B-1) If the poisoning reaction is extremely slow relative to diffusion of the poison, what is the value of the internal effectiveness factor of the poisonous molecule for this case? (B-2) If the poisoning reaction is rapid relative to diffusion of the poison, the outer periphery of the catalyst particle will be completely poisoned while the inner pore will be completely free of poison. The thickness of the poisoned shell grows with prolonged exposure to poison molecules until the pellet is completely deactivated. During the poisoning process, the boundary between active and deactivated regions is relatively sharp. See the figure below. When a fraction a of the total catalyst surface has been deactivated by poison, a cylindrical region of length (aL) nearest the pore mouth will have no catalytic activity, while a region of length [(1-a)L] will be catalytically active. The concentration of A at the pore mouth is Cas, and at z = aL is CA. Express the internal effectiveness factor of the reactant A as a function of CAS, CA, a, L, De, ro and k". Poisoned pore mouth C = CAS 250 z = 0 aL C = CAT (1-0)L + Z=L
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


