Question: Consider the following compound. Develop all reasonable retrosynthetic analyses for this compound and any diastereomers that involves CC bond forming reactions alkyiation using alkynide anions
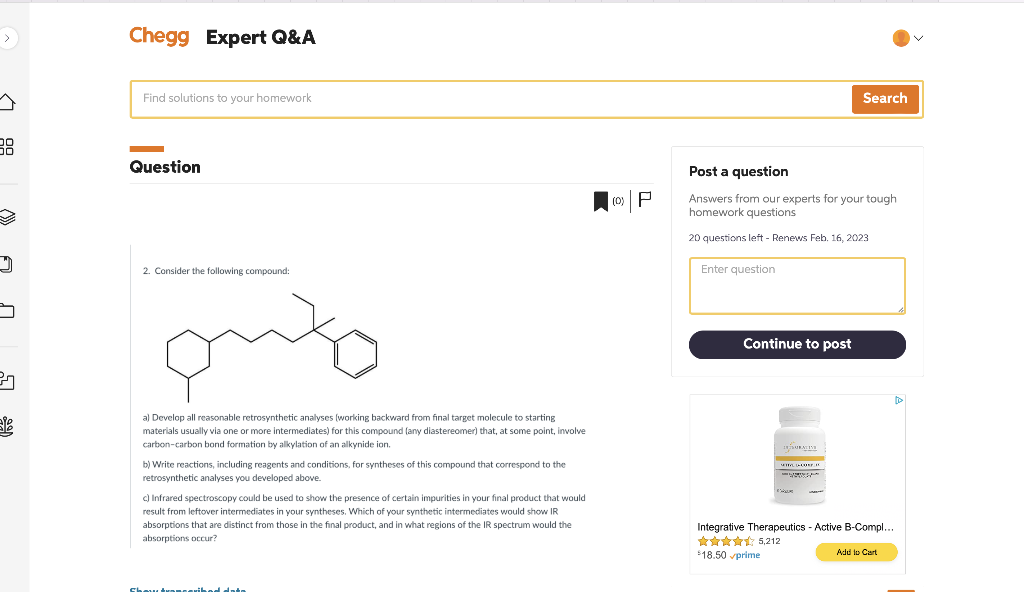 Consider the following compound. Develop all reasonable retrosynthetic analyses for this compound and any diastereomers that involves CC bond forming reactions alkyiation using alkynide anions starting with starting matenals that are no more than b carbons each.
Consider the following compound. Develop all reasonable retrosynthetic analyses for this compound and any diastereomers that involves CC bond forming reactions alkyiation using alkynide anions starting with starting matenals that are no more than b carbons each.
Question Post a question Answers from our experts for your tough homework questions 20 questions left - Renews Feb. 16, 2023 2. Consider the following compound: a) Develop all reasonable retrosynthetic analyses (working backward from final target molecule to starting materials usually via one or more intermediates) for this compound (any diastereomer) that, at some point, involve carbon-carbon bond formation by alkylation of an alkynide ion. b) Write reactions, including reagents and conditions, for syntheses of this compound that correspond to the retrosynthetic analyses you developed above. c) Infrared spectroscopy could be used to show the presence of certain impurities in your final product that would result from leftover intermediates in your syntheses. Which of your synthetic intermediates would show R absorptions that are distinct from those in the final product, and in what regions of the IR spectrum would the absorptions occur? Integrative Therapeutics - Active B-Compl
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


