Question: Consider the following concentration vs time plot for a one-step reaction involving three gases: A. B, & C: 0.03 0.02 0.01 B 0.00 100
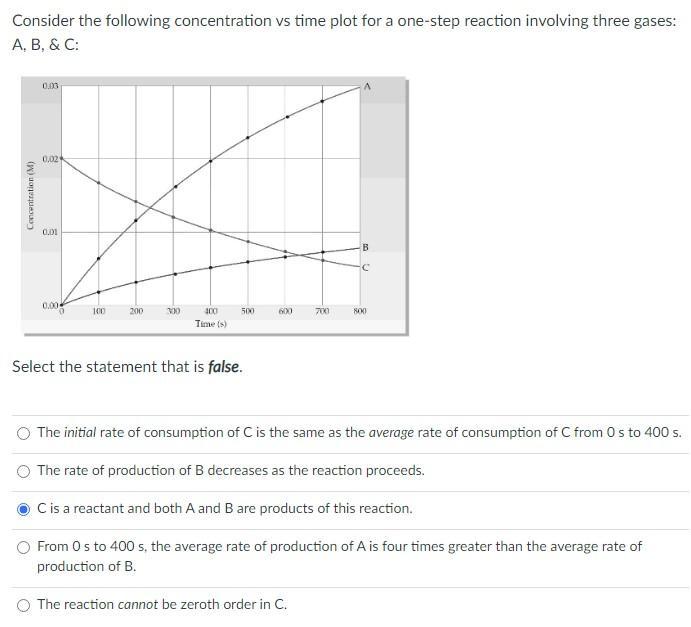
Consider the following concentration vs time plot for a one-step reaction involving three gases: A. B, & C: 0.03 0.02 0.01 B 0.00 100 200 300 400 500 600 700 S00 Time (s) Select the statement that is false. O The initial rate of consumption of C is the same as the average rate of consumption of C from 0 s to 400 s. O The rate of production of B decreases as the reaction proceeds. Cis a reactant and both A and B are products of this reaction. O From Os to 400 s, the average rate of production of A is four times greater than the average rate of production of B. O The reaction cannot be zeroth order in C. Concentration (M)
Step by Step Solution
3.42 Rating (152 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


