Question: 6. Consider the following concentration table. 0(8) 2.0 mol I (initial moles) C (change) E (equilibrium moles) 3 H(g) + 4.0 mol 2 NH3(g)
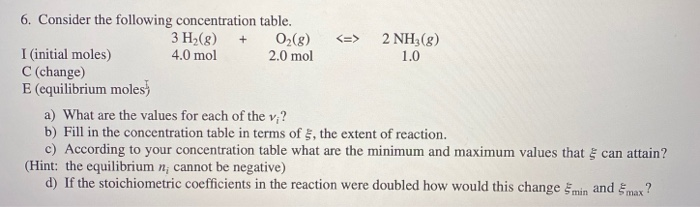
6. Consider the following concentration table. 0(8) 2.0 mol I (initial moles) C (change) E (equilibrium moles) 3 H(g) + 4.0 mol 2 NH3(g) 1.0 a) What are the values for each of the vi? b) Fill in the concentration table in terms of 5, the extent of reaction. c) According to your concentration table what are the minimum and maximum values that can attain? (Hint: the equilibrium n; cannot be negative) d) If the stoichiometric coefficients in the reaction were doubled how would this change min and max?
Step by Step Solution
3.45 Rating (161 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


