Question: Consider the following data on some weak acids and weak bases: acid base K K name formula name formula acetic acid HCH,CO2 1.8x10- methylamine CH
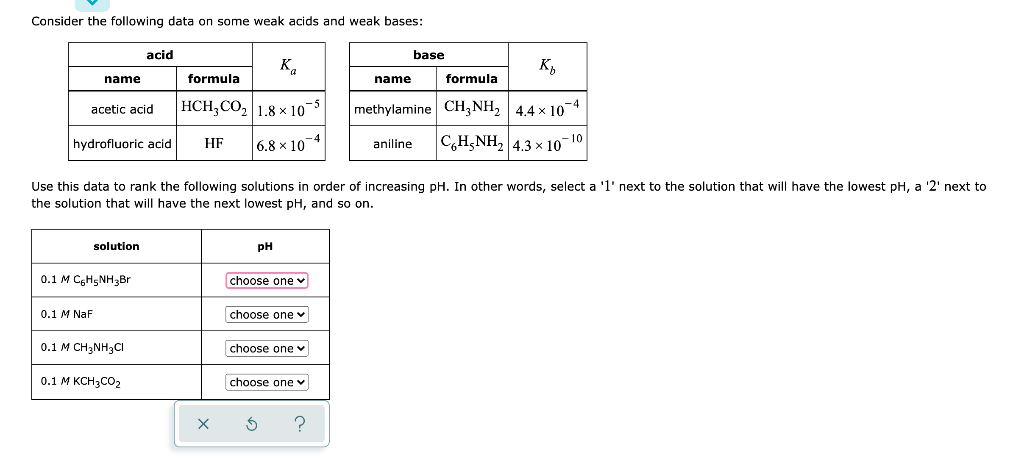
Consider the following data on some weak acids and weak bases: acid base K K name formula name formula acetic acid HCH,CO2 1.8x10- methylamine CH NH 4.4x 10-4 CH3NH2 4.3 x 10-10 hydrofluoric acid HF 6.8 x 10-4 aniline Use this data to rank the following solutions in order of increasing pH. In other words, select a 'l' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. solution pH 0.1 M C H5NH3Br choose one 0.1 M NaF choose one 0.1 M CH3NH2CI choose one 0.1 M KCH3CO2 choose one X
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


