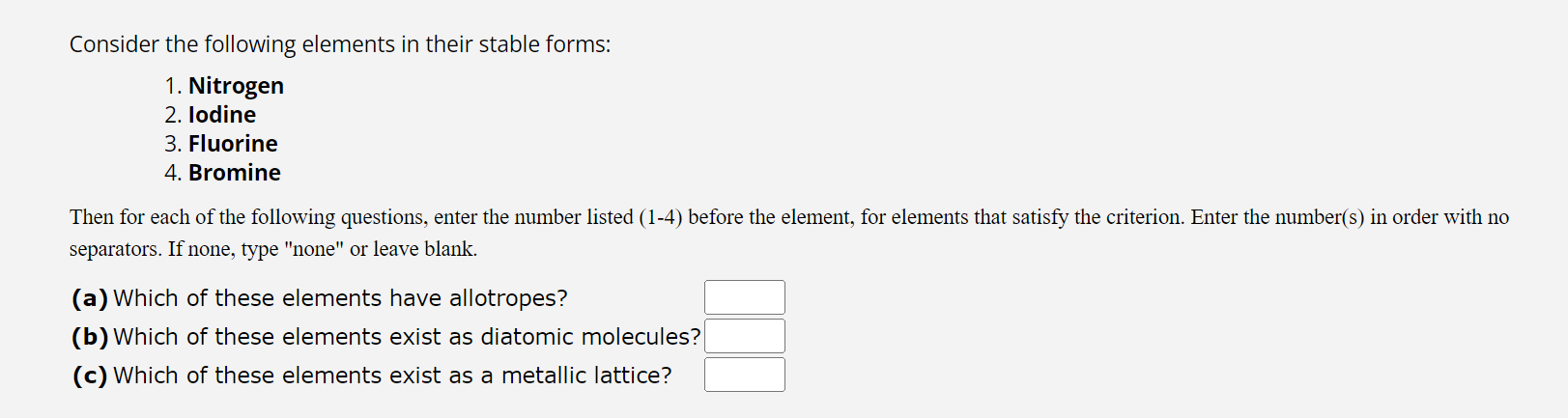
Question: Consider the following elements in their stable forms: Nitrogen lodine Fluorine Bromine Then for each of the following questions, enter the number listed ( 1
Consider the following elements in their stable forms:
Nitrogen
lodine
Fluorine
Bromine
Then for each of the following questions, enter the number listed before the element, for elements that satisfy the criterion. Enter the numbers in order with no
separators. If none, type "none" or leave blank.
a Which of these elements have allotropes?
b Which of these elements exist as diatomic molecules?
c Which of these elements exist as a metallic lattice?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


