Question: Consider the following net ionic equation for a redox reaction: H2O+2MnO4+SO322MnO42+SO42+2H+ (a) Which reactant gets oxidized? (b) Which reactant gets reduced? (c) Which reactant is
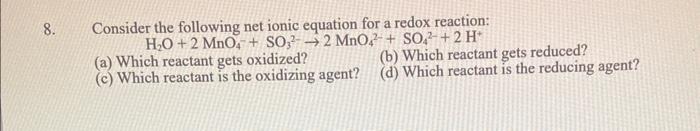
Consider the following net ionic equation for a redox reaction: H2O+2MnO4+SO322MnO42+SO42+2H+ (a) Which reactant gets oxidized? (b) Which reactant gets reduced? (c) Which reactant is the oxidizing agent? (d) Which reactant is the reducing agent
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


