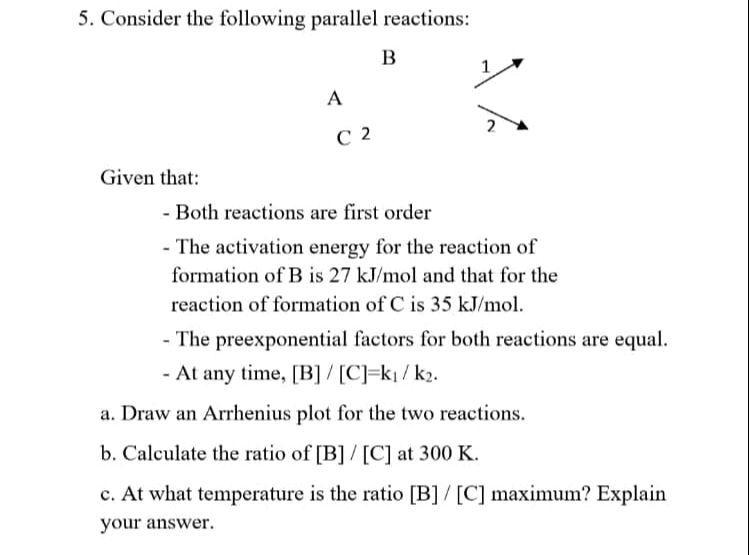
Question: Consider the following parallel reactions: B A C 2 Given that: Both reactions are first order The activation energy for the reaction of formation of
Consider the following parallel reactions:
B
A
C
Given that:
Both reactions are first order
The activation energy for the reaction of formation of B is and that for the reaction of formation of is
The preexponential factors for both reactions are equal.
At any time,
a Draw an Arrhenius plot for the two reactions.
b Calculate the ratio of at
c At what temperature is the ratio maximum? Explain your answer.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


