Question: Consider the following reaction: A -> P Through kinetic experiments, it was found that A has an order of 7 and k = 1.0 M
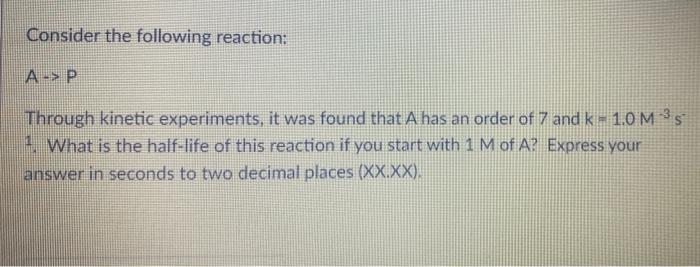
Consider the following reaction: A -> P Through kinetic experiments, it was found that A has an order of 7 and k = 1.0 M 35 1. What is the half-life of this reaction if you start with 1 M of A? Express your answer in seconds to two decimal places (XX.XX)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


