Question: Consider the following two reactions and write down the balanced chemical equations. Reaction 1: Formic acid (CH2O2, I) reacts with oxygen to produce liquid water
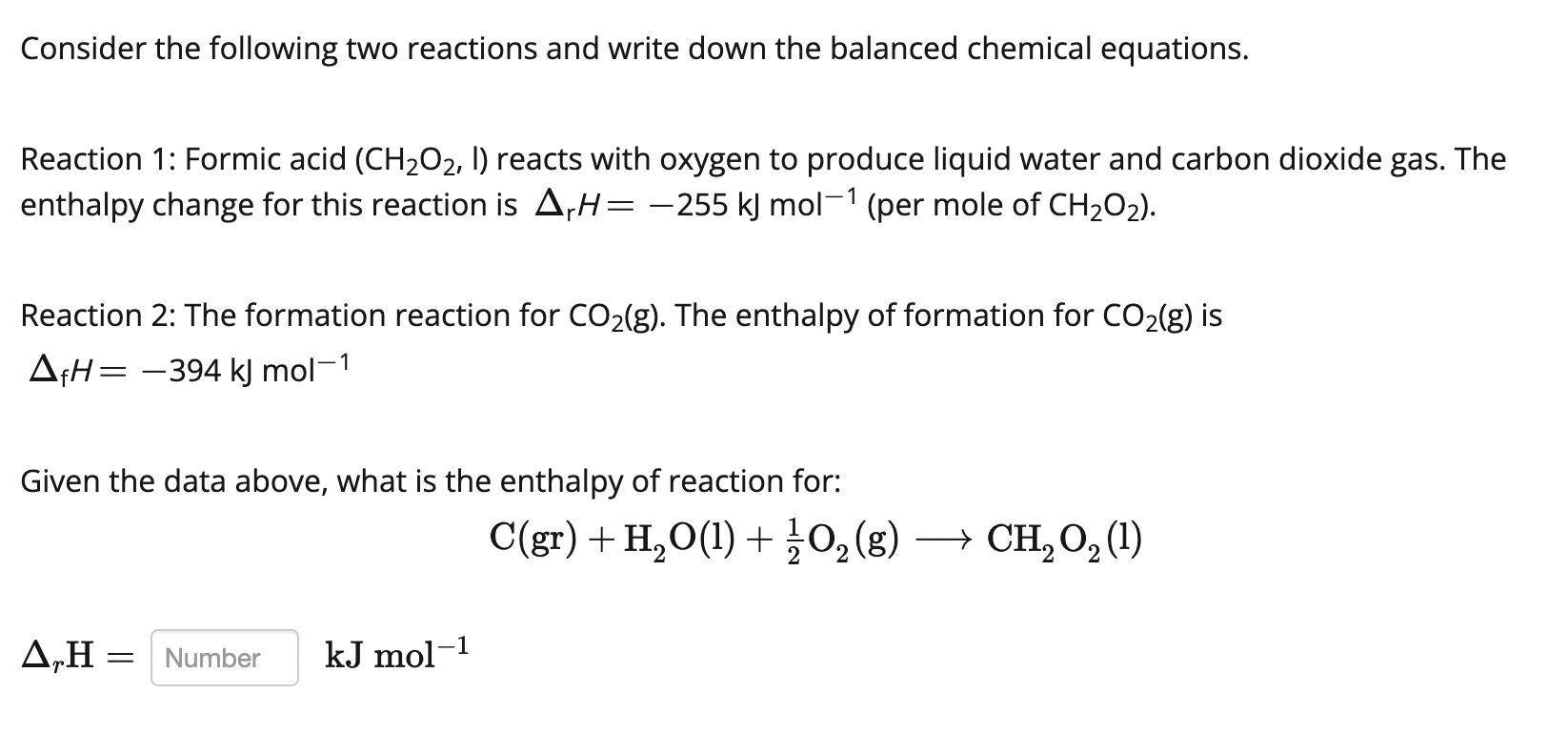
Consider the following two reactions and write down the balanced chemical equations. Reaction 1: Formic acid (CH2O2, I) reacts with oxygen to produce liquid water and carbon dioxide gas. The enthalpy change for this reaction is ArH= 255 kJ mol-1 (per mole of CH2O2). Reaction 2: The formation reaction for CO2(g). The enthalpy of formation for CO2(g) is AfH= 394 kJ mol-1 Given the data above, what is the enthalpy of reaction for: C(gr) + H2O(1) + O2(g) CH,02(1) ArH= Number = kJ mol-1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


