Question: Consider the irreversible gas phase reaction A+BC with rate expression r=kCACB Part a Assuming ideal gas behavior, derive an expression for the reactor volume required
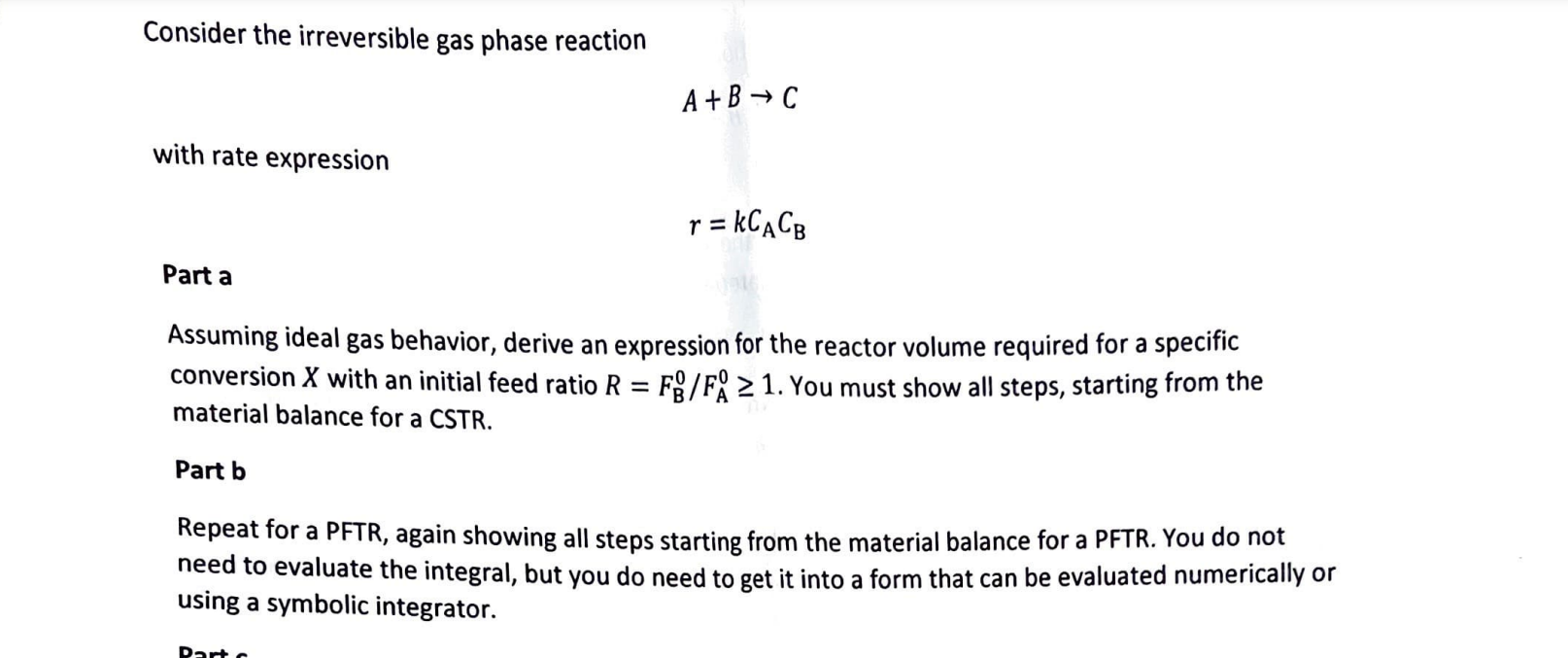
Consider the irreversible gas phase reaction A+BC with rate expression r=kCACB Part a Assuming ideal gas behavior, derive an expression for the reactor volume required for a specific conversion X with an initial feed ratio R=FB0/FA01. You must show all steps, starting from the material balance for a CSTR. Part b Repeat for a PFTR, again showing all steps starting from the material balance for a PFTR. You do not need to evaluate the integral, but you do need to get it into a form that can be evaluated numerically using a symbolic integrator
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


