Question: Consider the methanol-ethanol system to form an ideal solution. Do the following: 1. Construct the P-ry diagram at 100 C. 2. Construct the T-ry diagram
Consider the methanol-ethanol system to form an ideal solution. Do the following: 1. Construct the P-ry diagram at 100 C. 2. Construct the T-ry diagram at 1.2 bar. 3. What is the bubble point and dew point for a mixture containing 20% methanol by mole? 4. Applying Raoult's law, predict the temperature of the methanol-ethanol system at 101.325 kPa and the composition of the vapor phase. Construct the T - ry graph for this mixture. Compare the results of your model with the set of experimental data shown below at 101,325 kPa. Is this system correctly modified by the Law of Raoult? Justify your answer. 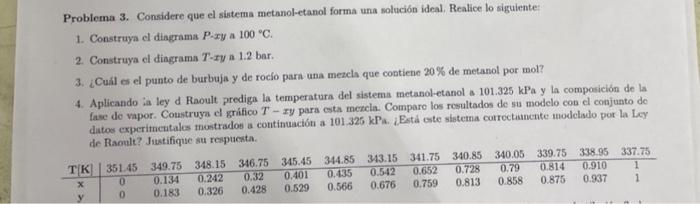
Problema 3. Considere que el sistema metanol-etanol forma una solucin ideal. Realice lo siguiente: 1. Construya el diagrama P-ry a 100 C. 2. Construya el dingrama T-ryn 1.2 bar. 3. Cul es el punto de burbuja y de rocio para una mezcla que contiene 20% de metanol por mol? 4. Aplicando la ley d Raoult prediga la temperatura del sistema metanol-etanol 101.325 kPa y la composicin de la fase de vapor. Coustruya el grfico T-ry para esta metcia. Compare los resultados de su modelo con el conjunto de datos experimentales mostrados a continuacin a 101.325 kPa. Est este sistema correctamente modelado por la Ley de Raoult? Justifique su respuesta TK 351.45 349.75 348.15 346.75 345.45 344.85 313.15 341.75 340.85 340.05 339.75 338.95 337.75 0.134 0.242 0.32 0.401 0.135 0.542 0.652 0.728 0.79 0.814 0.910 1 0.183 0.326 0.428 0.529 0.566 0.676 0.759 0.813 0.858 0.875 0.937 1 0 0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


