Question: Consider the neutralization reaction between magnesium hydroxide and hydrochloric acid. 2HCl(aq)+Mg(OH)2(aq)MgCl2(aq)+2H2O( Calculate the mass of Mg(OH)2 required to completely neutralize 200mL of 0.70MHCl. mass: g
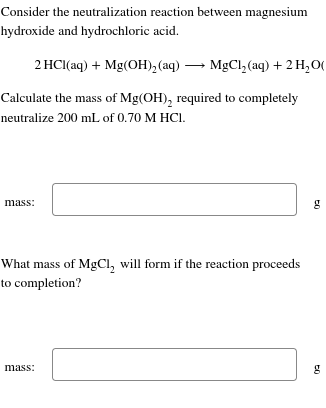
Consider the neutralization reaction between magnesium hydroxide and hydrochloric acid. 2HCl(aq)+Mg(OH)2(aq)MgCl2(aq)+2H2O( Calculate the mass of Mg(OH)2 required to completely neutralize 200mL of 0.70MHCl. mass: g What mass of MgCl2 will form if the reaction proceeds to completion
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


