Question: Consider the reaction 2N05 + 4NO2 + 02, the experimental rate law for the decomposition of N20s being v = k[N205]. For this reaction, it
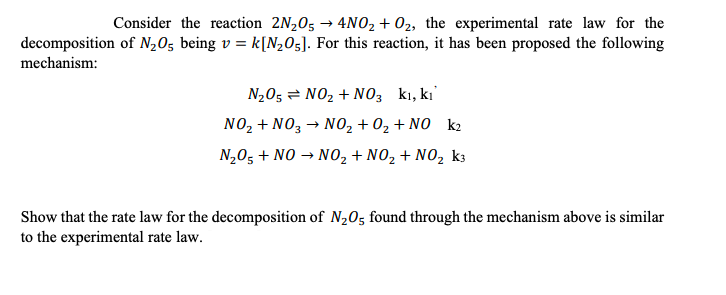
Consider the reaction 2N05 + 4NO2 + 02, the experimental rate law for the decomposition of N20s being v = k[N205]. For this reaction, it has been proposed the following mechanism: N205 = NO2 + NO3 ki, ki NO2 + NO3 NO2 + O2 + NO k2 N205 + NO + NO2 + NO2 + NO, k3 Show that the rate law for the decomposition of N2O5 found through the mechanism above is similar to the experimental rate law
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


