Question: Consider the reaction. Attempt A(aq) = 2 B(aq) K. = 7.96 x 10-6 at 500 K If a 2.10 M sample of A is heated
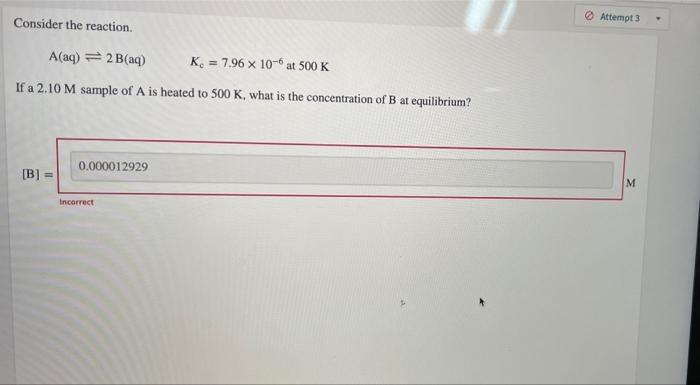
Consider the reaction. Attempt A(aq) = 2 B(aq) K. = 7.96 x 10-6 at 500 K If a 2.10 M sample of A is heated to 500 K, what is the concentration of B at equilibrium? 0.000012929 [B] = M Incorrect
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


