Question: Consider the reaction: I 2 ( g ) + C l 2 ( g ) h a r r 2 ICl ( g ) K
Consider the reaction: ICl
Enough ICI was added to reach a partial pressure of atm in a previously evacuated vessel. After the reaction reached equilibrium, the pressure of the was atm. Complete an ICE diagram to show find the equilibrium pressures of each species and then use those values to calculate the value of the equilibrium constant.
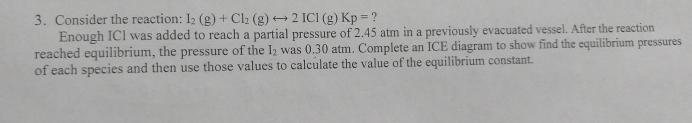
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


