Question: Please help me with this question! I will upvote you Thanks! For the following chemical reaction, calculate the standard heat of reaction in two ways
Please help me with this question!
I will upvote you Thanks!
For the following chemical reaction, calculate the standard heat of reaction in two ways using data from Table B.1: (i) from standard heats of formation and (ii) from standard heats of combustion. If you can only calculate the standard heat of reaction using one method, say so. a. Chlorination of ethane to produce chloroethane C 2 H 6 (g) + Cl 2 (g) C 2 H 5 Cl (g) + HCl (g)
b. Complete combustion of gaseous n-propane C 3 H 8 (g) + 10 O2 (g) 3 CO2 (g) + 4 H 2 O (l)
c. Burning of ammonia to form nitric oxide 4NH 3 (g) + 5 O2 (g) 4 NO (g) + 6 H 2 O (v)
B1. Table
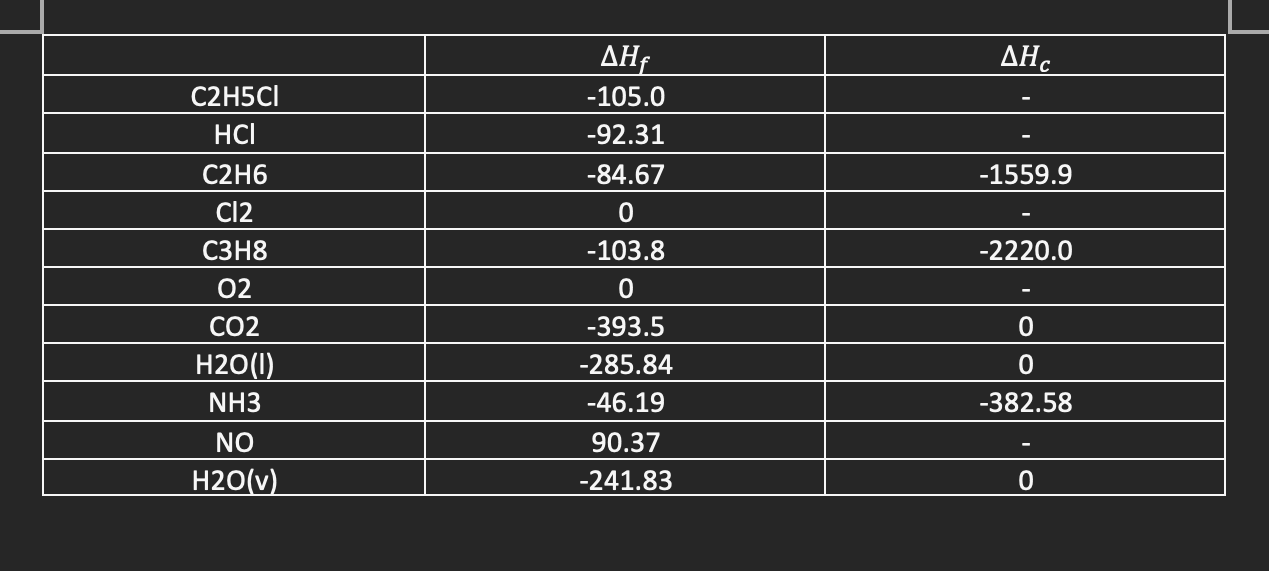
\begin{tabular}{|c|c|c|} \hline & Hf & Hc \\ \hline C2H5Cl & -105.0 & - \\ \hline HCl & -92.31 & - \\ \hline C2H6 & -84.67 & -1559.9 \\ \hline Cl2 & 0 & - \\ \hline C3H8 & -103.8 & -2220.0 \\ \hline O2 & 0 & - \\ \hline CO2 & -393.5 & 0 \\ \hline H2O(l) & -285.84 & 0 \\ \hline NH3 & -46.19 & -382.58 \\ \hline NO & 90.37 & - \\ \hline H2O(v) & -241.83 & 0 \\ \hline \end{tabular} \begin{tabular}{|c|c|c|} \hline & Hf & Hc \\ \hline C2H5Cl & -105.0 & - \\ \hline HCl & -92.31 & - \\ \hline C2H6 & -84.67 & -1559.9 \\ \hline Cl2 & 0 & - \\ \hline C3H8 & -103.8 & -2220.0 \\ \hline O2 & 0 & - \\ \hline CO2 & -393.5 & 0 \\ \hline H2O(l) & -285.84 & 0 \\ \hline NH3 & -46.19 & -382.58 \\ \hline NO & 90.37 & - \\ \hline H2O(v) & -241.83 & 0 \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


