Question: Consider the reactions between the two unknown diatomic molecules (X2 and Y2). Consider the following reaction at 1000K : XY(g)X(g)+Y(g) What is the equilibrium constant?
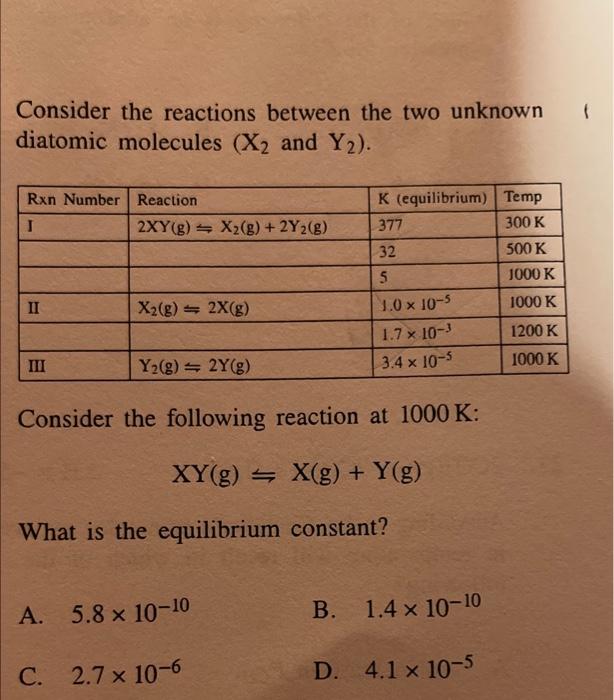
Consider the reactions between the two unknown diatomic molecules (X2 and Y2). Consider the following reaction at 1000K : XY(g)X(g)+Y(g) What is the equilibrium constant? A. 5.81010 B. 1.41010 C. 2.7106 D. 4.1105
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


