Question: Consider the rectangular plate (battery) as shown in the figure below. The battery is generating heat at the rate of Q gen W/m 3 .
Consider the rectangular plate (battery) as shown in the figure below. The battery is generating heat at the rate of Qgen W/m3. Different thermal boundary conditions are imposed on the four sides of the battery as indicated (h is the heat transfer coefficient and Tfluid is the temperature of the cooling fluid) such that steady state conditions are maintained.
The left and right sides of the plate are hot and are losing heat to the surrounding fluid
Note that its top and bottom sides are insulated thereby reducing this to a heat conduction problem in 1-D (along the x-axis as shown).
Determine the maximum heat generation rate Qgen within the battery for the conditions shown such that the maximum temperature within the battery does not exceed 320 K.
A. Choose the appropriate transport equation for energy conservation and reduce the equation by eliminating all the zero terms.
B. Solve the differential equation that you obtained in part (a) to get a general solution for the temperature variation inside the plate as a function of "x" and two arbitrary constants C1 and C2 .
C. Employing your boundary conditions associated with this problem, evaluate the values of the constants C1 and C2 .
D. Using the temperature profile that you obtained in Part (c), determine the maximum allowable value of Qgen W/m3.
E. Using the results obtained from your hand calculations, show that your solution corresponds to steady-state conditions by comparing the fluxes at the right and left walls and the heat generated within the battery (you can assume the battery has dimensions of 1m x 1m x 1m) .
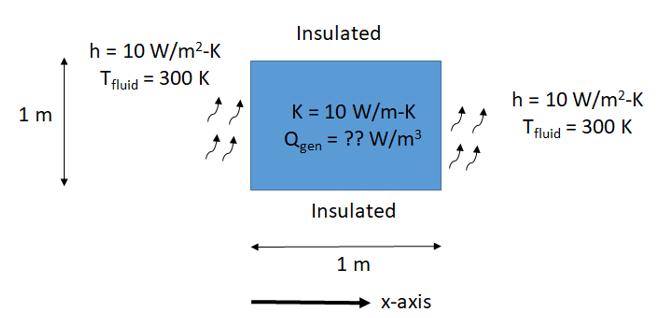
1 m h = 10 W/m-K Tfluid = 300 K Insulated K = 10 W/m-K Qgen = ?? W/m Insulated 1 m x-axis h = 10 W/m-K Tfluid = 300 K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


