Question: Consider the rigid, adiabatic box below. The box is separated into two compartments by a frictionless partition, one side containing CO2 and the other
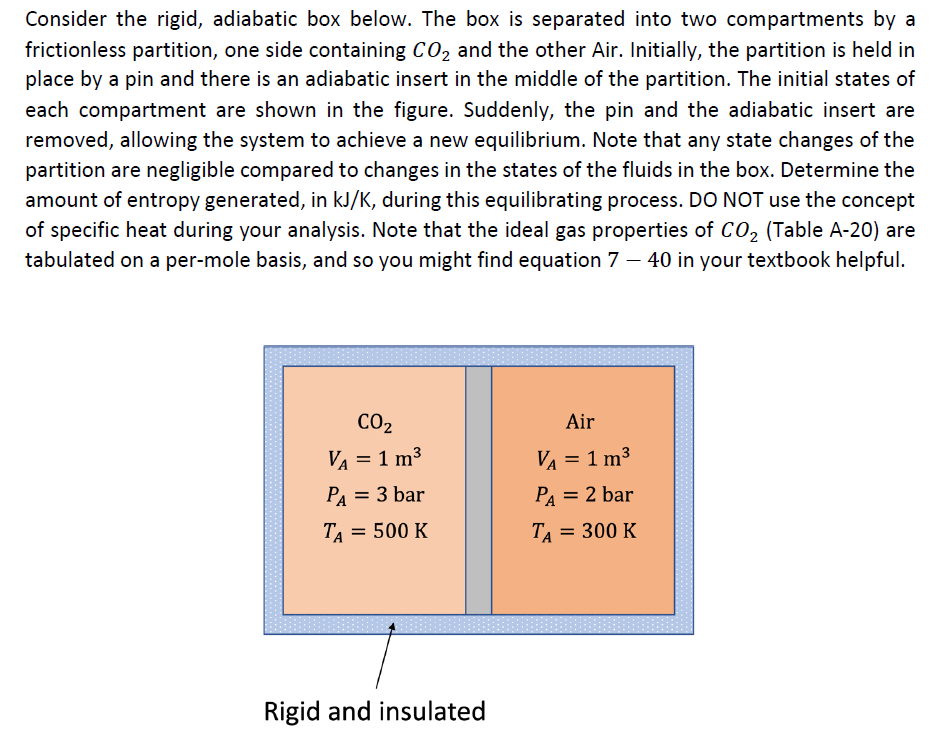
Consider the rigid, adiabatic box below. The box is separated into two compartments by a frictionless partition, one side containing CO2 and the other Air. Initially, the partition is held in place by a pin and there is an adiabatic insert in the middle of the partition. The initial states of each compartment are shown in the figure. Suddenly, the pin and the adiabatic insert are removed, allowing the system to achieve a new equilibrium. Note that any state changes of the partition are negligible compared to changes in the states of the fluids in the box. Determine the amount of entropy generated, in kJ/K, during this equilibrating process. DO NOT use the concept of specific heat during your analysis. Note that the ideal gas properties of CO2 (Table A-20) are tabulated on a per-mole basis, and so you might find equation 7 40 in your textbook helpful. CO2 Air V = 1 m VA V = 1 m PA = 3 bar PA = 2 bar TA = 500 K TA = 300 K Rigid and insulated
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


