Question: Consider the same liquid phase irreversible reaction as described in Problem 1 . Here, the reaction is to occur in two identical CSTRs as shown
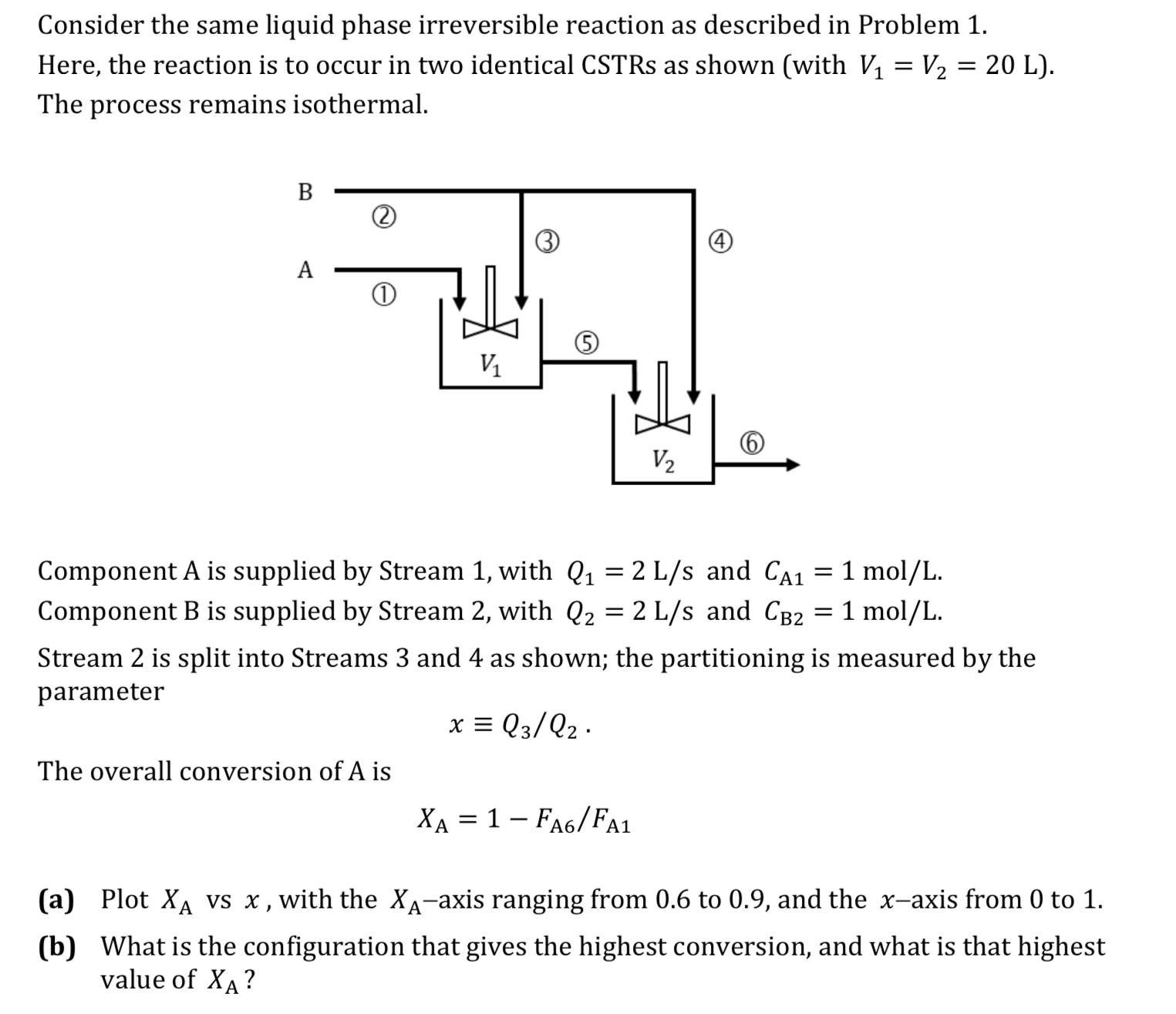
Consider the same liquid phase irreversible reaction as described in Problem Here, the reaction is to occur in two identical CSTRs as shown with The process remains isothermal.
Component A is supplied by Stream with and Component B is supplied by Stream with and
Stream is split into Streams and as shown; the partitioning is measured by the parameter
The overall conversion of is
a Plot vs with the axis ranging from to and the axis from to
b What is the configuration that gives the highest conversion, and what is that highest value of
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


