Question: > Consider the second-order reaction A + 4B, where A and B are ideal gases. The reaction takes place in an isothermal and isobaric flow
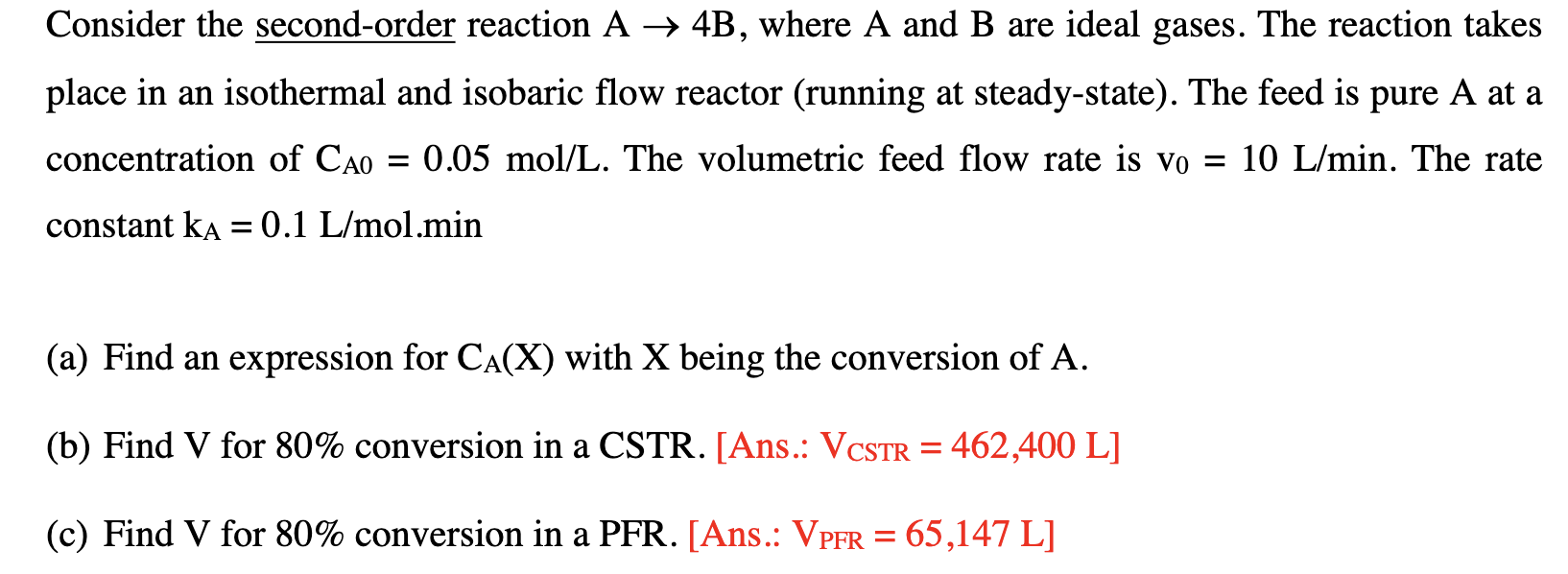
> Consider the second-order reaction A + 4B, where A and B are ideal gases. The reaction takes place in an isothermal and isobaric flow reactor (running at steady-state). The feed is pure A at a concentration of Cao = 0.05 mol/L. The volumetric feed flow rate is vo = 10 L/min. The rate constant ka = 0.1 L/mol.min - (a) Find an expression for CA(X) with X being the conversion of A. (b) Find V for 80% conversion in a CSTR. [Ans.: VCSTR = 462,400 L] a = (c) Find V for 80% conversion in a PFR. [Ans.: VPFR = 65,147 L] a
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


