Question: Consider the two tanks as shown in (Figure 11-2), which are connected in series. The first tank has a volume of salt water V, with
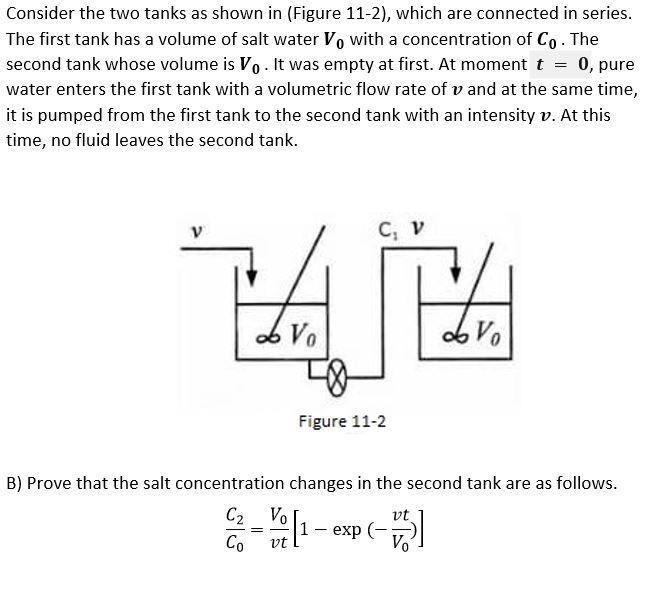

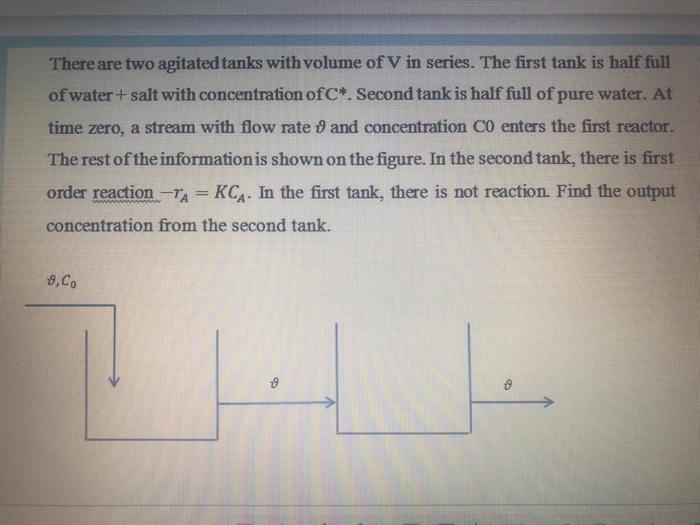
Consider the two tanks as shown in (Figure 11-2), which are connected in series. The first tank has a volume of salt water V, with a concentration of Co. The second tank whose volume is V. It was empty at first. At moment t = 0, pure water enters the first tank with a volumetric flow rate of v and at the same time, it is pumped from the first tank to the second tank with an intensity v. At this time, no fluid leaves the second tank. V ZAJE doro Figure 11-2 B) Prove that the salt concentration changes in the second tank are as follows. C2 VO vt Co vo - Vi [1-ex HALA vt WEE Markeu oul 10.00 P Flag question MA M - 1 71 7 A o A There are two agitated tanks with volume of V in series. The first tank is half full of water + salt with concentration ofC*. Second tank is half full of pure water. At time zero, a stream with flow rate 9 and concentration C0 enters the first reactor. The rest of the information is shown on the figure. In the second tank, there is first order reaction -TA = KCA. In the first tank, there is not reaction. Find the output concentration from the second tank. - & Co 9
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


