Question: Consider the zeroth order decomposition of A with an initial concentration of 4.5M. If k=0.0270s1, what is the concentration of A after 40 seconds? Your
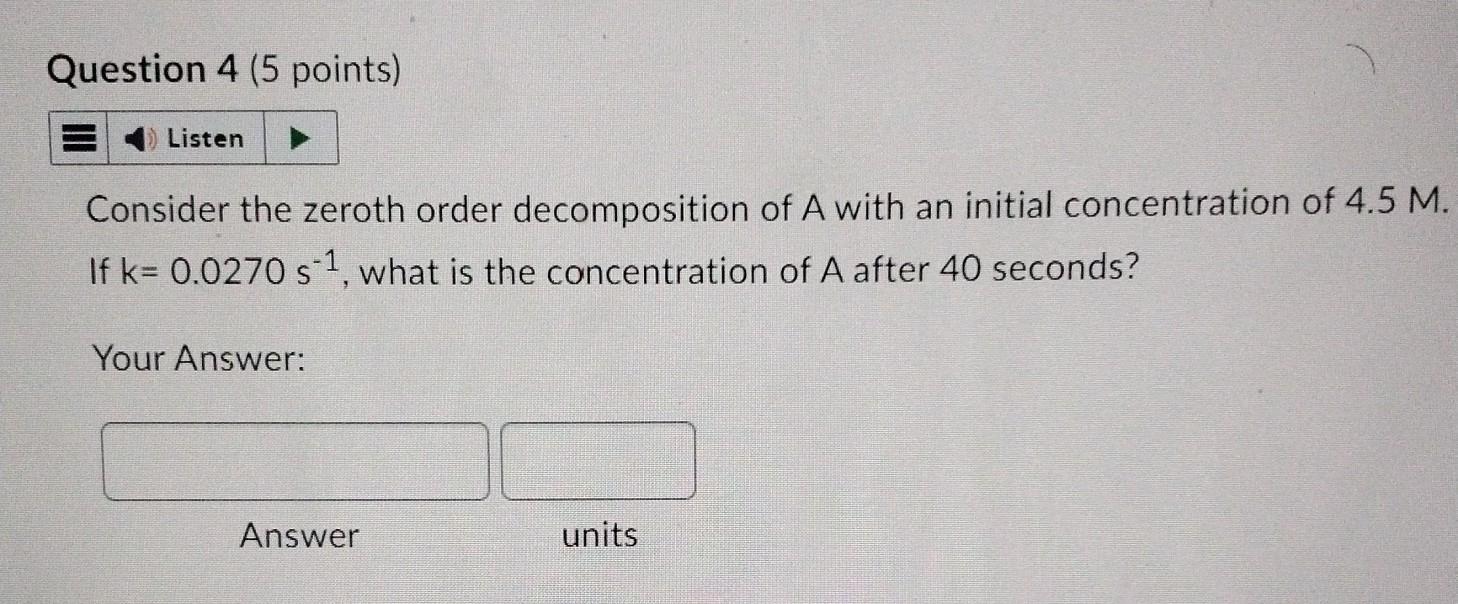
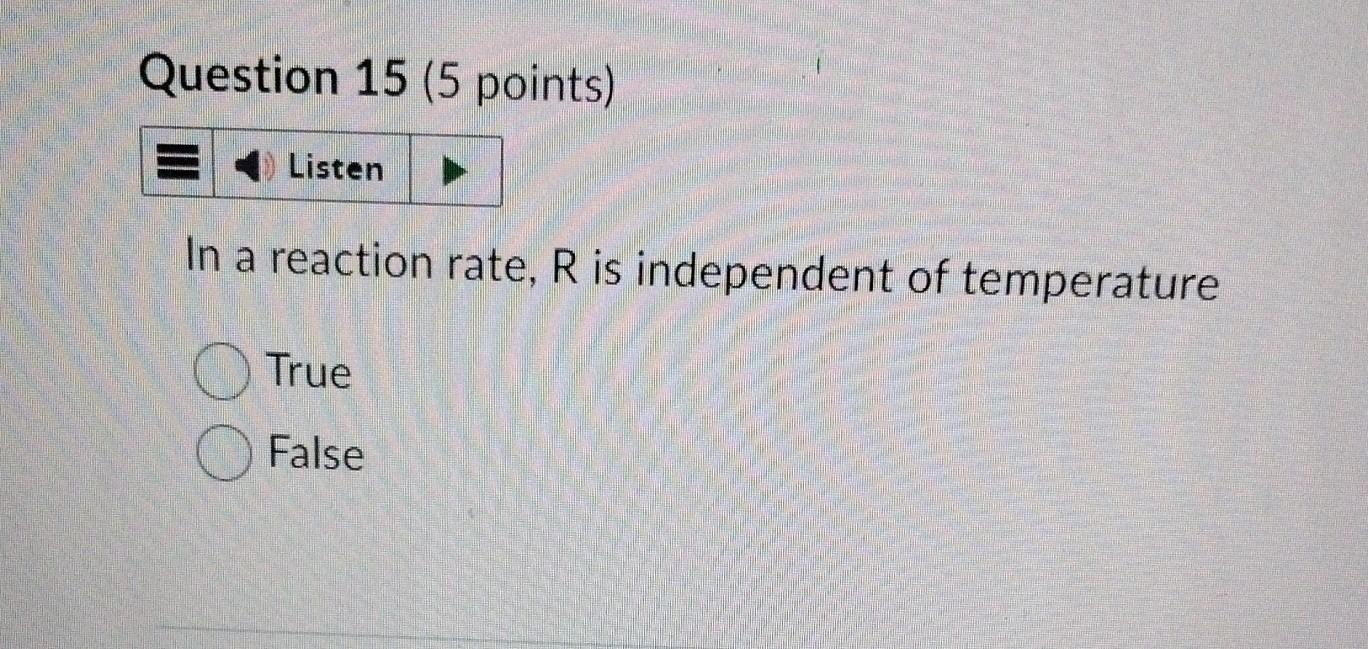
Consider the zeroth order decomposition of A with an initial concentration of 4.5M. If k=0.0270s1, what is the concentration of A after 40 seconds? Your Answer: Answer units In a reaction rate, R is independent of temperature True False
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


