Question: Consider this reaction: 2HI(g)H2(g)+I2(g) At a certain temperature it obeys this rate law. rate=(0.567M1,5=1)[HI]2 Suppose a vessel contains HI at a concentration of 1.27M. Calculate
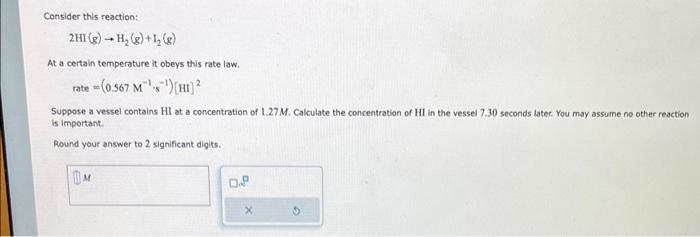
Consider this reaction: 2HI(g)H2(g)+I2(g) At a certain temperature it obeys this rate law. rate=(0.567M1,5=1)[HI]2 Suppose a vessel contains HI at a concentration of 1.27M. Calculate the concentration of HI in the vessel 7.30 seconds iater. You may assume no other reaction is important. Round your answer to 2 significant digits
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


