Question: Consider two boxes, each of which is filled with ideal gases. Consider that the two boxes are combined. 1. Under what condition or conditions
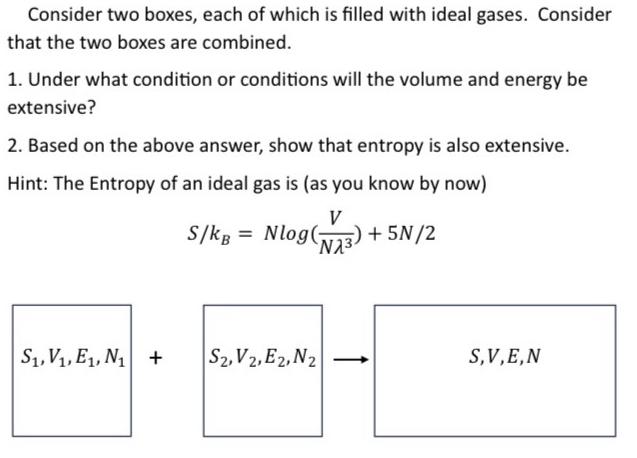
Consider two boxes, each of which is filled with ideal gases. Consider that the two boxes are combined. 1. Under what condition or conditions will the volume and energy be extensive? 2. Based on the above answer, show that entropy is also extensive. Hint: The Entropy of an ideal gas is (as you know by now) V NA +5N/2 S/kg Nlog(; = anaa + aran-[ S, V, E, N S2, V2, E2, N2 S,V,E,N
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


