Question: Consider two products that may be processed in a single 50 cubic foot blender that has a product contact surface area of 750 dm2. Highly-potent
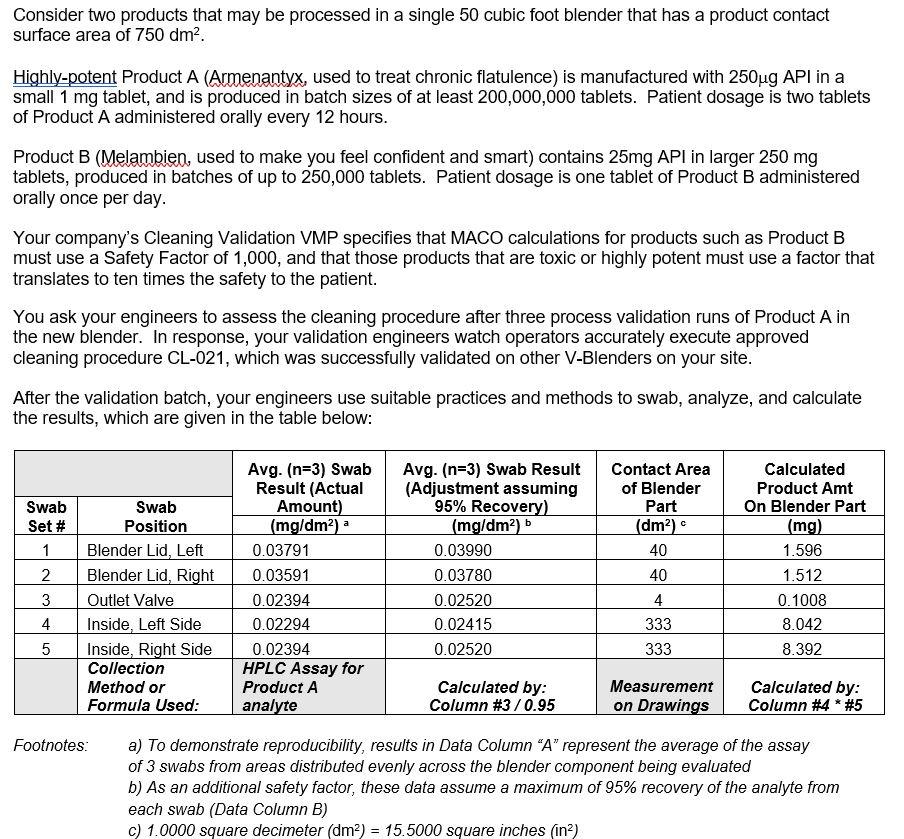



Consider two products that may be processed in a single 50 cubic foot blender that has a product contact surface area of 750 dm2. Highly-potent Product A (Armenantyx, used to treat chronic flatulence) is manufactured with 250ug API in a small 1 mg tablet, and is produced in batch sizes of at least 200,000,000 tablets. Patient dosage is two tablets of Product A administered orally every 12 hours. Product B (Melambien, used to make you feel confident and smart) contains 25mg API in larger 250 mg tablets, produced in batches of up to 250,000 tablets. Patient dosage is one tablet of Product B administered orally once per day. Your company's Cleaning Validation VMP specifies that MACO calculations for products such as Product B must use a Safety Factor of 1,000, and that those products that are toxic or highly potent must use a factor that translates to ten times the safety to the patient. You ask your engineers to assess the cleaning procedure after three process validation runs of Product A in the new blender. In response, your validation engineers watch operators accurately execute approved cleaning procedure CL-021, which was successfully validated on other V-Blenders on your site. After the validation batch, your engineers use suitable practices and methods to swab, analyze, and calculate the results, which are given in the table below: Swab Set # 1 Contact Area of Blender Part (dm) 40 Avg. (n=3) Swab Result (Actual Amount) (mg/dm2) 0.03791 0.03591 0.02394 0.02294 0.02394 HPLC Assay for Product A analyte Avg. (n=3) Swab Result (Adjustment assuming 95% Recovery) (mg/dm2) 0.03990 0.03780 0.02520 0.02415 0.02520 Swab Position Blender Lid, Left Blender Lid, Right Outlet Valve Inside, Left Side Inside, Right Side Collection Method or Formula Used: 2 Calculated Product Amt On Blender Part (mg) 1.596 1.512 0.1008 8.042 8.392 3 4 5 40 4 333 333 Calculated by: Column #3/0.95 Measurement on Drawings Calculated by: Column #4 * #5 Footnotes: a) To demonstrate reproducibility, results in Data Column "A" represent the average of the assay of 3 swabs from areas distributed evenly across the blender component being evaluated b) As an additional safety factor, these data assume a maximum of 95% recovery of the analyte from each swab (Data Column B) C) 1.0000 square decimeter (dm) = 15.5000 square inches (in) 1) (14 points total) Assuming these are the only 2 products run on a piece of equipment whose cleaning you will need to validate: a) (1 points per box) List in the table below the values for each of the MACO calculation parameters. Clearly document the calculations you perform for each parameter so I can grade for partial credit (note: I can't grade a correct #full credit, either!). Don't forget to give the units! PARAMETER DTD PRODUCTA PRODUCTB MBS SF MDW b) (1 points per formula) In the space below, carefully print the formulas for calculating the MACO of Product A as the contaminant, and for the MACO of Product B as the contaminant: Formula: MACO (A in B) Formula: MACO (B in A) c) (2 points each box) Using the parameter values recorded above in Question 1a) and the formula you recorded above, calculate the two MACOs and record them in the table below. Remember, show your calculations. Don't forget to be careful with the units! MACO (A in B) MACO (B in A) 2) (9 points total) The following questions refer to the correct answers for Part 1, which you have hopefully provided. a) (4 points) Which of the two products is "worst case" for patient safety - Product A, or Product B? Why? b) (5 points) Let's say for another product, Product C, that it can have contamination of Product B with a MACO of 10kg. When the cleaning study is performed, the results show that the blender retained an amount of Product B equal to 90% of this MACO value. Does this mean that the blender is safe for use without re-cleaning (from a patient/consumer perspective)? Does this mean that the blender is clean? Why or why not? 3) (12 points total) The following questions refer to the validation data presented earlier. The cleaning validation has three acceptance criteria for validating the procedure: a) Equipment must be "visibly clean. Note: your company defines this based on the "average person's" visual LOD, which in turn was based on research studies that have approximated this limit to be 100ug spread evenly over a 4in2 area). b) Total contamination of the equipment must be less than the MACO for that product as a contaminant, based on cumulative swab results. c) No individual location of the cleaned equipment may have >35.Opg/dm2 per swab. In this company, Cleaning Validation would pass only if all three of these are met. Explain which of the criteria pass (if any) and which fail (if any) and conclude whether the validation passes or fails. Justify your answer by comparing the actual obtained results vs. the acceptance criteria








