Question: Relationship between the reaction quotient and the equilibrium constant The equilibrium concentration of each component depends on the initial conditions, the direction in which

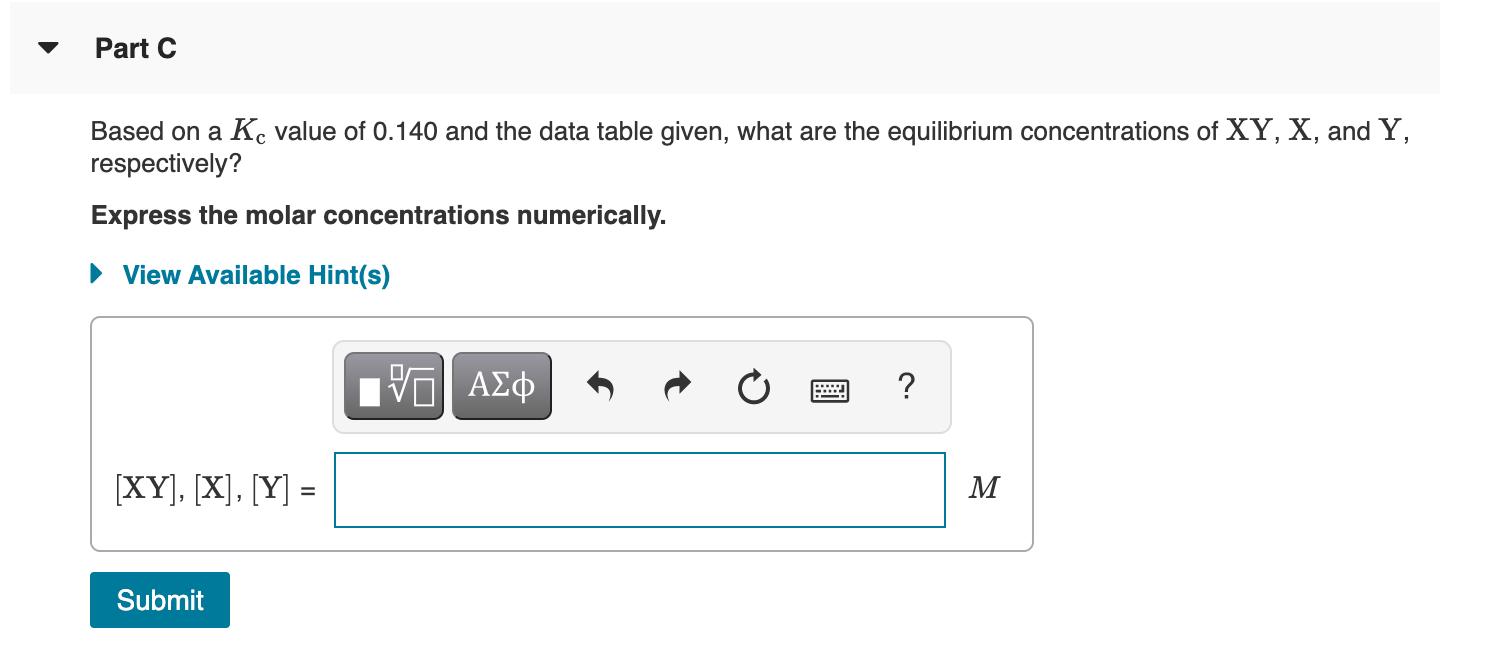
Relationship between the reaction quotient and the equilibrium constant The equilibrium concentration of each component depends on the initial conditions, the direction in which the reaction proceeds to attain equilibrium, and the magnitude of the K value. If K > Q for the initial concentrations, the net reaction will proceed forward to produce more products and increase Q. If K < Q for the initial concentrations, the net reaction will proceed in reverse to produce more reactants and decrease Q. (Figure 1) Part A Based on a K. value of 0.140 and the initial concentrations given in the table, determine in which direction the net reaction will proceed to attain equilibrium. Initial concentrations (M) Mixture XY [] | [Y] A 0.100 0.500 0.100 0.100 C 0.200 0.300 0.300 Drag the items into the appropriate bins. View Available Hint(s) Learning Goal: To determine equilibrium concentrations from initial conditions. The reversible reaction XY(aq) = X(aq) +Y(aq) has a reaction quotient Qc defined as Figure 1 of 1 K KQ K Reaction Reaction Equilibrium forms forms products reactants Part C Based on a K. value of 0.140 and the data table given, what are the equilibrium concentrations of XY, X, and Y, respectively? Express the molar concentrations numerically. View Available Hint(s) H [XY], [X], [Y] = M Submit Based on a K, value of 0.140 and the given data table, what are the equilibrium concentrations of XY, X, and Y, respectively? Express the molar concentrations numerically. View Available Hint(s) ? [XY], [X], [Y] = M Your submission doesn't have the correct number of answers. Answers should be separated with a comma. No credit lost. Try again. Submit Previous Answers
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


