Question: Constants | Periodic Table In 1922, two German scientists, Otto Stern and Walter Gerlach, studied the pattern of deflection of neutral atoms Consider a beam
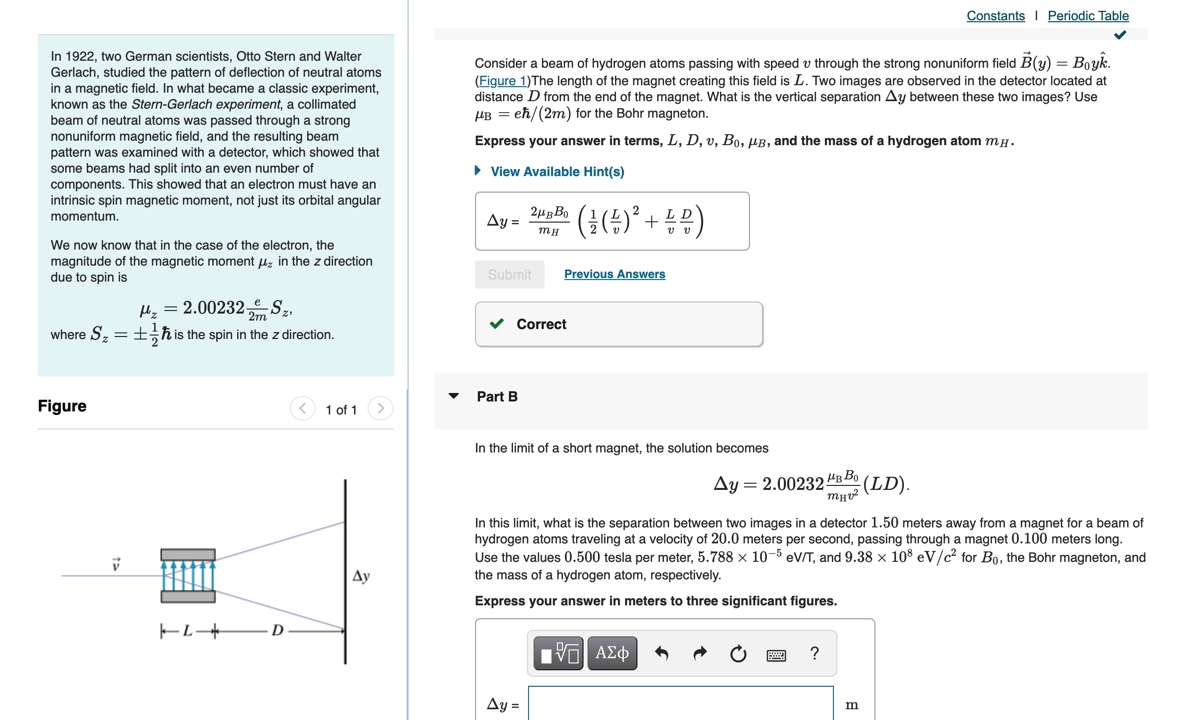
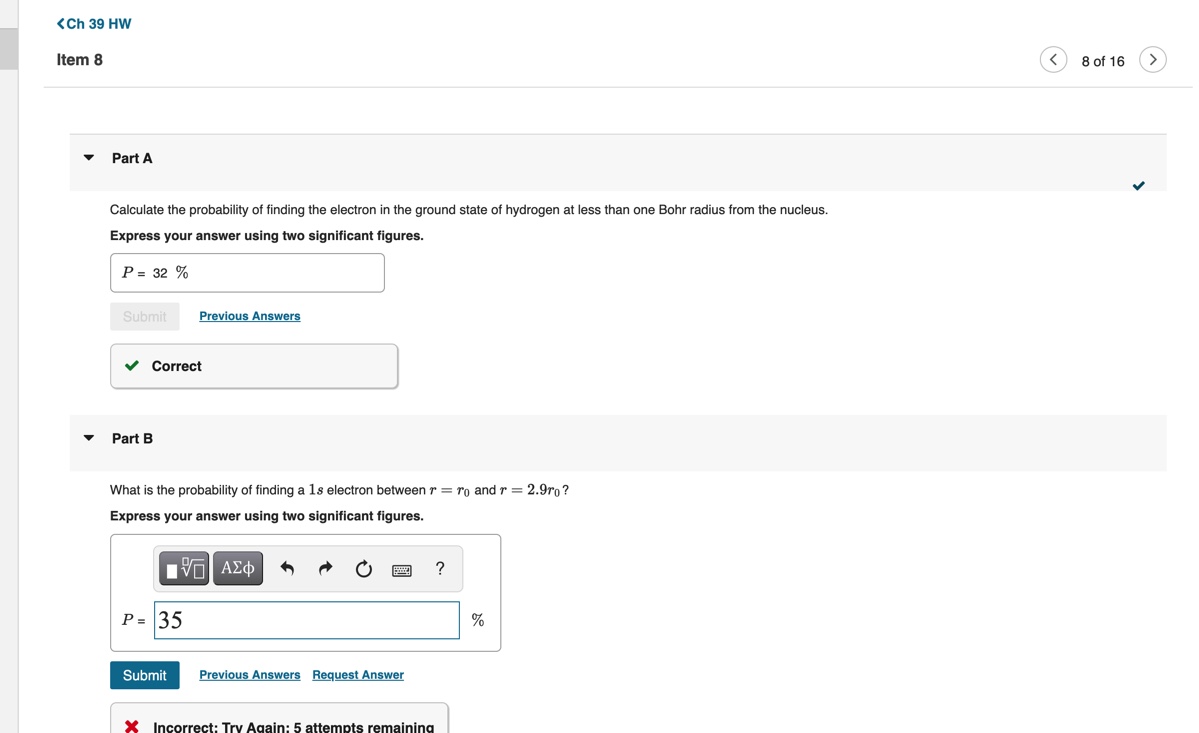
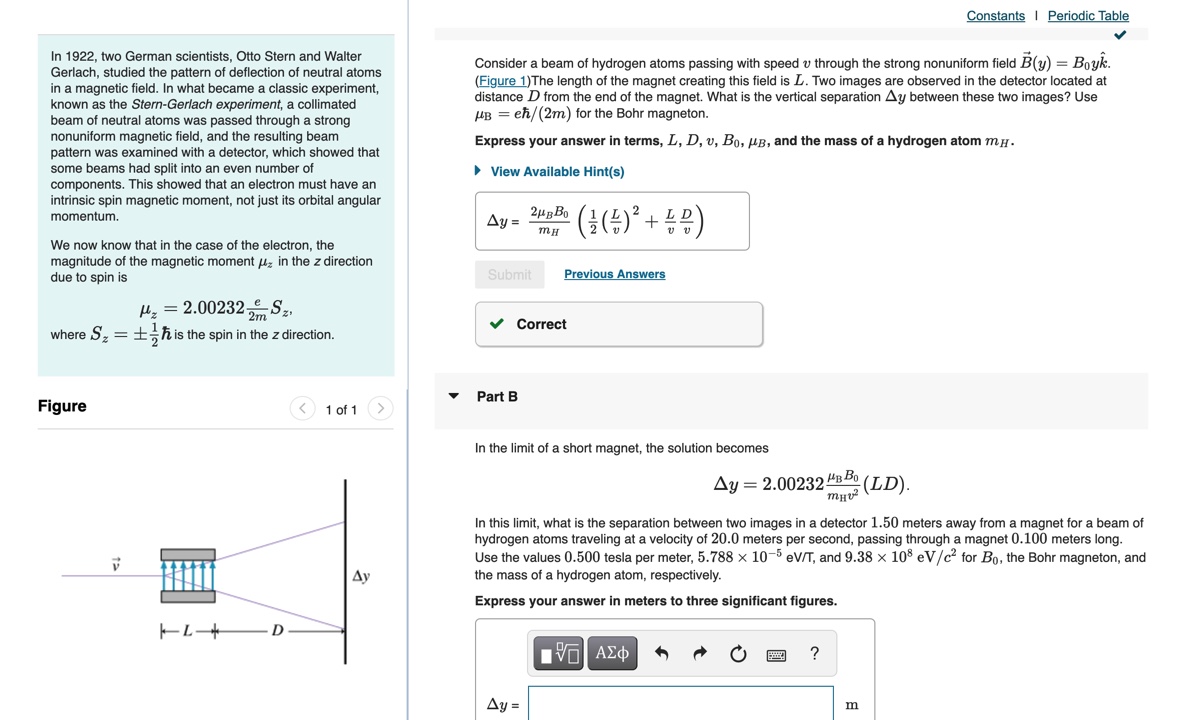
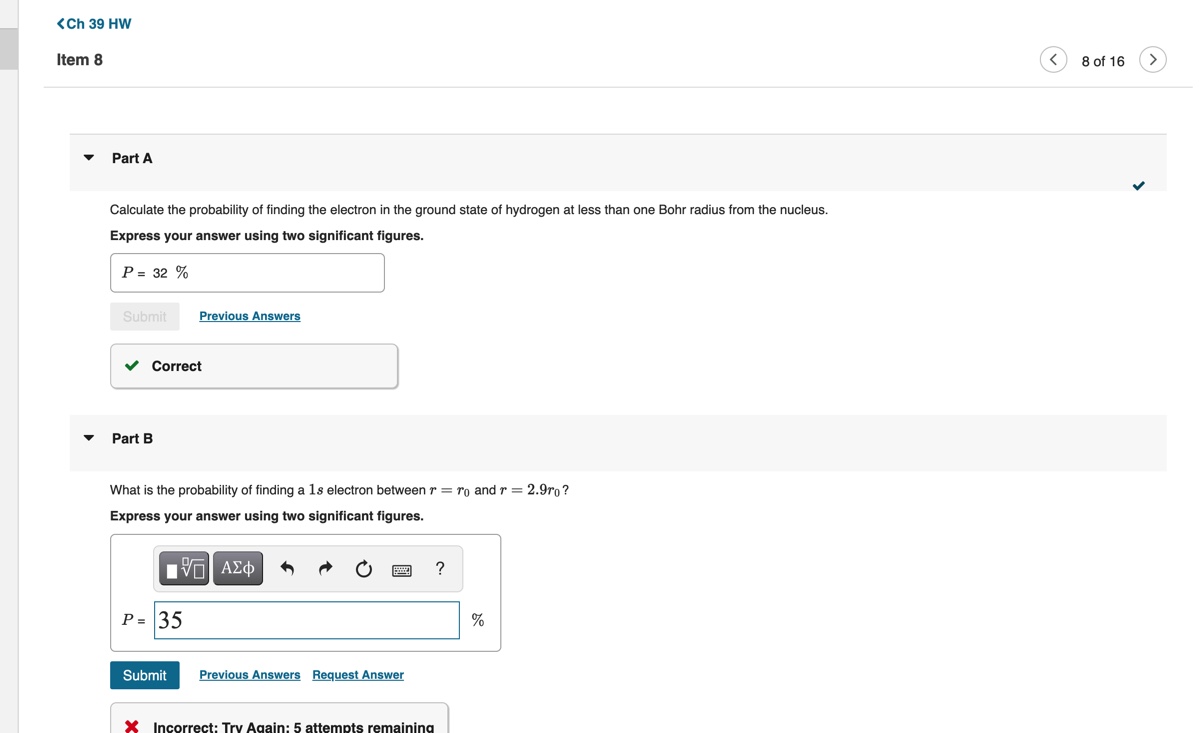
Constants | Periodic Table In 1922, two German scientists, Otto Stern and Walter Gerlach, studied the pattern of deflection of neutral atoms Consider a beam of hydrogen atoms passing with speed v through the strong nonuniform field B(y) = Boyk. in a magnetic field. In what became a classic experiment, (Figure 1) The length of the magnet creating this field is L. Two images are observed in the detector located at known as the Stern-Gerlach experiment, a collimated distance D from the end of the magnet. What is the vertical separation Ay between these two images? Use beam of neutral atoms was passed through a strong UB = eh/(2m) for the Bohr magneton. nonuniform magnetic field, and the resulting beam Express your answer in terms, L, D, v, Bo, AB, and the mass of a hydrogen atom my. pattern was examined with a detector, which showed that some beams had split into an even number of > View Available Hint(s) components. This showed that an electron must have an intrinsic spin magnetic moment, not just its orbital angular momentum. Ay = 2HB BO ( # ( # ) 2 + BB ) We now know that in the case of the electron, the magnitude of the magnetic moment / in the z direction due to spin is Submit Previous Answers Hz = 2.00232 Correct where S= = _- h is the spin in the z direction. Part B Figure Part A v Calculate the probability of finding the electron in the ground state of hydrogen at less than one Bohr radius from the nucleus. Express your answer using two significant figures. P = 32 % Submit Previous Answers Correct Part B What is the probability of finding a 1s electron between r = ro and r = 2.9ro? Express your answer using two significant figures. IVO AEd OF ? P = 135 Submit Previous Answers Request
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


